GHGH Formula - C14H26O11 - Over 100 million chemical compounds
Por um escritor misterioso
Last updated 10 abril 2025

GHGH contains total 51 atom(s); 26 Hydrogen atom(s), 14 Carbon atom(s), and 11 Oxygen atom(s). Learn more about GHGH chemical formula at Mol-Instincts.
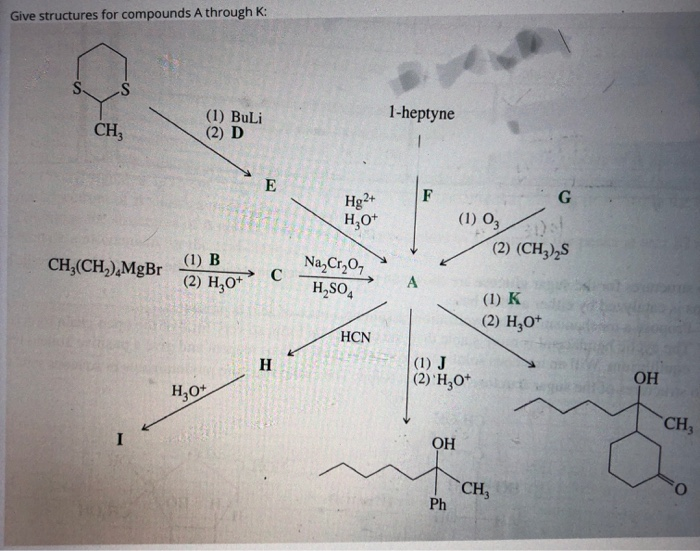
Solved Give structures for compounds A through K: 1-heptyne

GHGH Formula - C14H26O11 - Over 100 million chemical compounds

Chem 1201 exam 1 1 .docx - Calculate the answer with proper significant figures. A 88.678 78.77 167.45 B 3.45 / 4.8999 - 0.4 0.3 C 600 6.00 –

a 3178 b 6714 c 671 d 7 Question 46 Galactose is a compound with the following
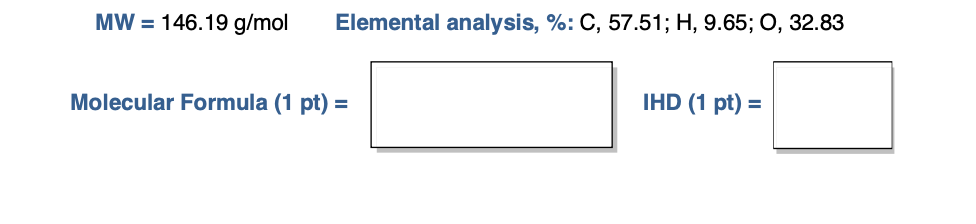
Solved MW = 146.19 g/mol Elemental analysis, %: C, 57.51; H

Hydroquinone is an organic compound commonly used as a photographic developer. It has a molecular weight of
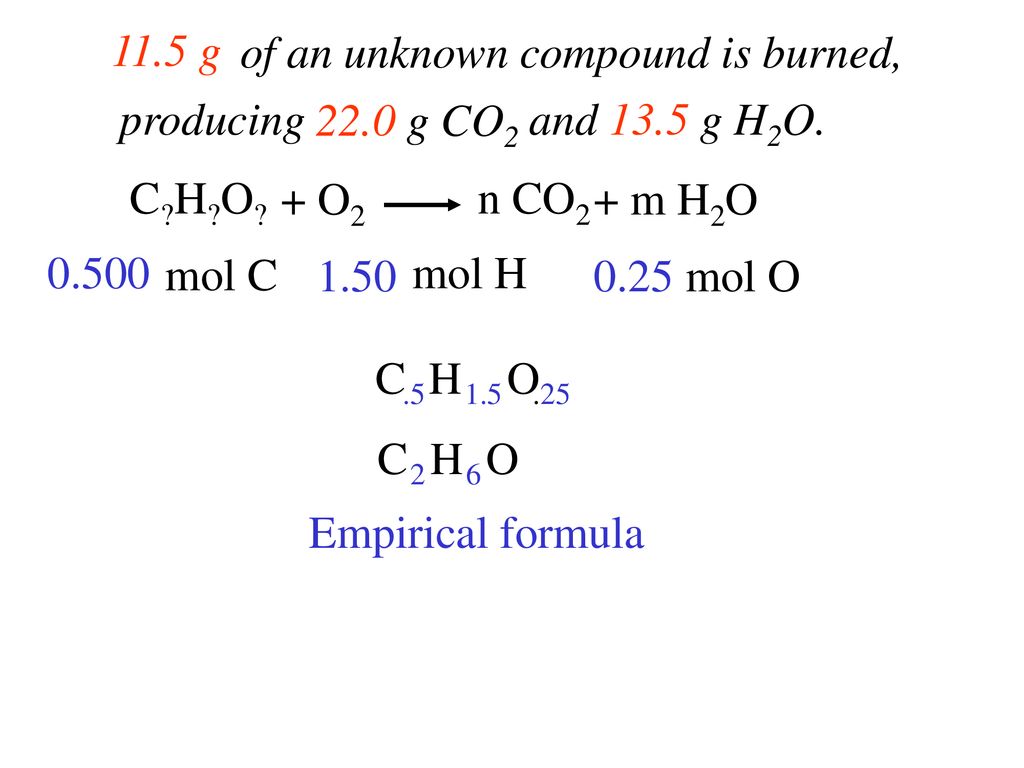
Molecular Formula number and type of atoms covalent compounds - ppt download

A compound with a known molecular weight (146.99 g/mol) that contains only C, H, and Cl was studied by combustion analysis. When a 0.367g sample was combusted, 0.659g of CO2 and 0.0892g

A compound with a known molecular weight (146.99 g/mol) that contains only C, H, and Cl was studied by combustion analysis. When a 0.367g sample was combusted, 0.659g of CO2 and 0.0892g
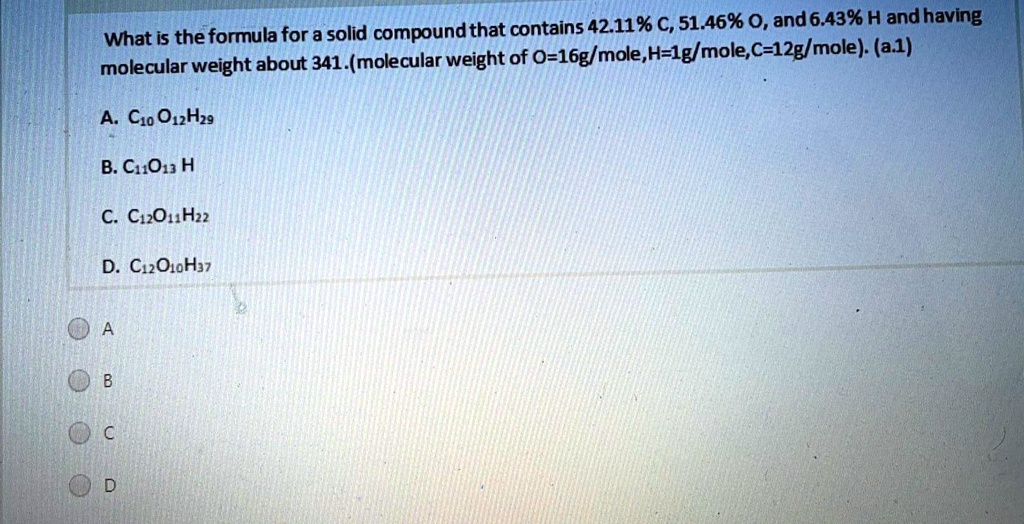
SOLVED: What is the formula for a solid compound that contains 42.11% C, 51.46% O, and 6.43% H and having a molecular weight of about 341 (molecular weight of O-16g/mole, H-1g/mole, C-12g/mole): (
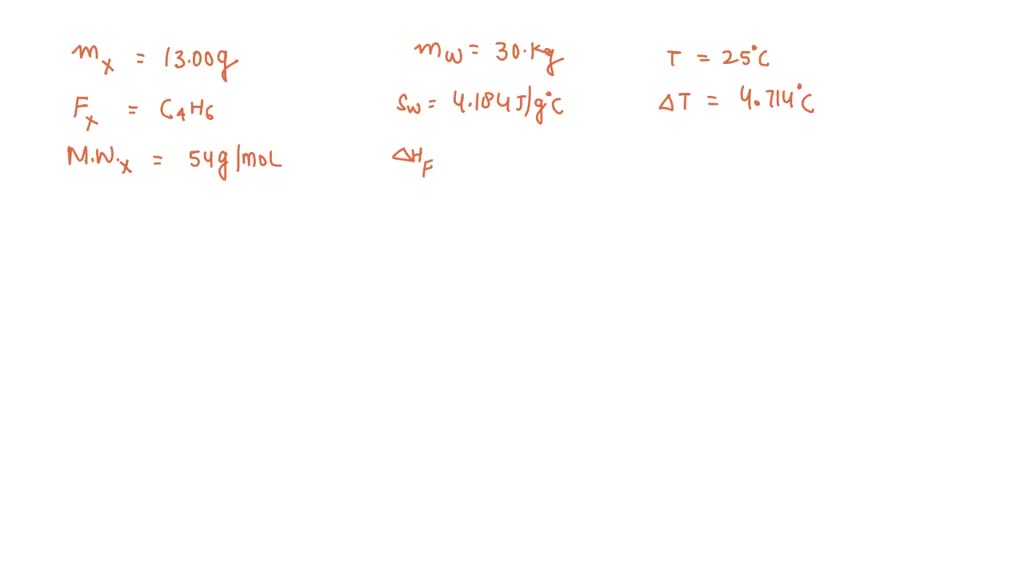
SOLVED: 13.00 g of Compound X with molecular formula C4H6 are burned in constant-pressure calorimeter containing 30.00 kg of water at 25 %C The temperature of the water is observed to rise
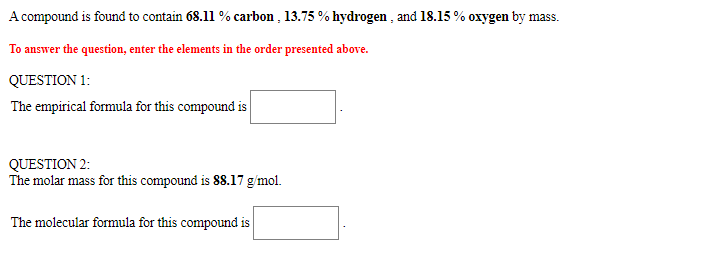
Solved A compound is found to contain 2.270 % hydrogen
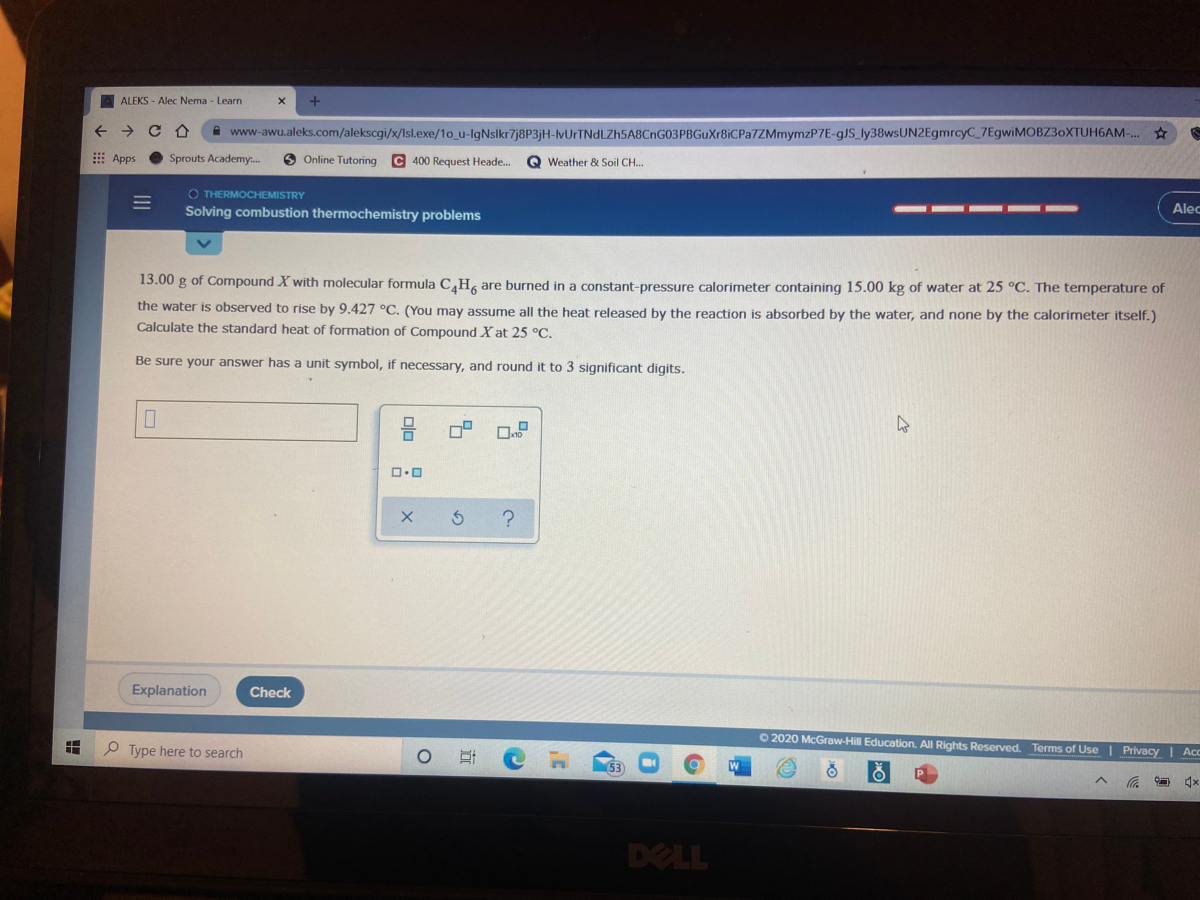
Answered: 13.00 g of Compound X with molecular…
Recomendado para você
-
 Tiverton GHGH GH PERFUME FOR WOMEN 3.4 OZ / 100 ML EAU DE PARFUM SPAY10 abril 2025
Tiverton GHGH GH PERFUME FOR WOMEN 3.4 OZ / 100 ML EAU DE PARFUM SPAY10 abril 2025 -
 Tiverton GHGH GH COLOGNE FOR MEN 3.4 OZ / 100 ML EAU DE PARFUM SPRAY10 abril 2025
Tiverton GHGH GH COLOGNE FOR MEN 3.4 OZ / 100 ML EAU DE PARFUM SPRAY10 abril 2025 -
Steam Workshop::ghgh10 abril 2025
-
 Stream Emőke Ambrus Listen to ghgh playlist online for free on10 abril 2025
Stream Emőke Ambrus Listen to ghgh playlist online for free on10 abril 2025 -
 Ghgh GIF - Ghgh - Discover & Share GIFs10 abril 2025
Ghgh GIF - Ghgh - Discover & Share GIFs10 abril 2025 -
 GHGH Summit for young girls across Monterey County10 abril 2025
GHGH Summit for young girls across Monterey County10 abril 2025 -
ghgh ichiro_10 abril 2025
-
 1 Free Ghgh music playlists10 abril 2025
1 Free Ghgh music playlists10 abril 2025 -
 Pixilart - ghgh by JACKJACK123410 abril 2025
Pixilart - ghgh by JACKJACK123410 abril 2025 -
 Ghgh (ghgh0328) - Profile10 abril 2025
Ghgh (ghgh0328) - Profile10 abril 2025
você pode gostar
-
 Fullmetal ALCHEMIST Mobile in 202310 abril 2025
Fullmetal ALCHEMIST Mobile in 202310 abril 2025 -
 Andrea Botez on X: we will one day take down GothamChess! But congratulations and deserved @GothamChess 👏 / X10 abril 2025
Andrea Botez on X: we will one day take down GothamChess! But congratulations and deserved @GothamChess 👏 / X10 abril 2025 -
 My Little Pony: Twilight Sparkle 2021 V110 abril 2025
My Little Pony: Twilight Sparkle 2021 V110 abril 2025 -
Key & BPM for Mommy Long Legs by Endigo, Maya Fennec10 abril 2025
-
 Tower of Fantasy codes (December 2023)10 abril 2025
Tower of Fantasy codes (December 2023)10 abril 2025 -
 Anime BOCCHI THE ROCK! hitori bocchi T-shirt Summer women men Short Sleeve Tees10 abril 2025
Anime BOCCHI THE ROCK! hitori bocchi T-shirt Summer women men Short Sleeve Tees10 abril 2025 -
Happy Wheels: The Series: Episode 2 - Friday Night Races on Vimeo10 abril 2025
-
 Thor: Ragnarok': The 15 Biggest Marvel Easter Eggs10 abril 2025
Thor: Ragnarok': The 15 Biggest Marvel Easter Eggs10 abril 2025 -
 Video Oscars 2021 predictions: Peter Travers on who will win, who10 abril 2025
Video Oscars 2021 predictions: Peter Travers on who will win, who10 abril 2025 -
 FIFA Expands Club World Cup, Adds Women's Competition10 abril 2025
FIFA Expands Club World Cup, Adds Women's Competition10 abril 2025


