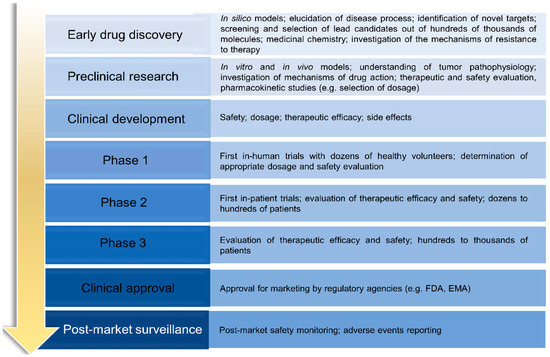
Early Safety Assessment - Drug Discovery and Development Based on
Por um escritor misterioso
Last updated 15 abril 2025

The drug candidate faces numerous efficacy and safety hurdles before moving forward to clinical testing. Here at the UPDDI we recognize the need for early identification of potential human toxicity and pharmacokinetic issues by creating a unique human liver microphysiological systems platform for drug testing before implementing preclinical animal testing.

Drug discovery - Wikipedia

Health economic considerations for early drug discovery - Drug
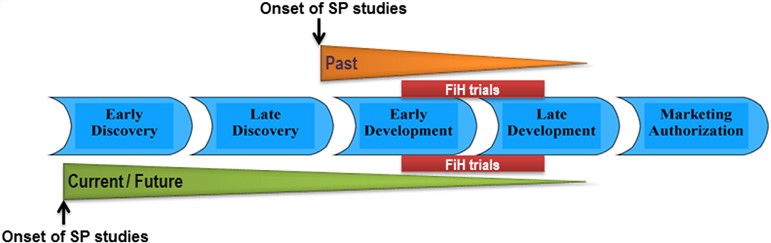
Supplemental Safety Pharmacology Studies - Creative Biolabs
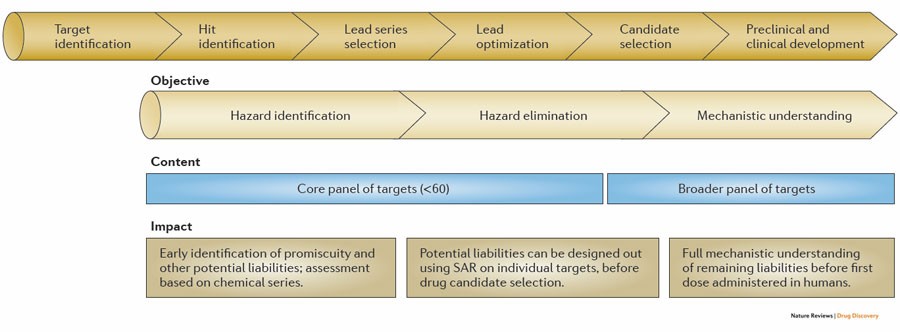
Reducing safety-related drug attrition: the use of in vitro
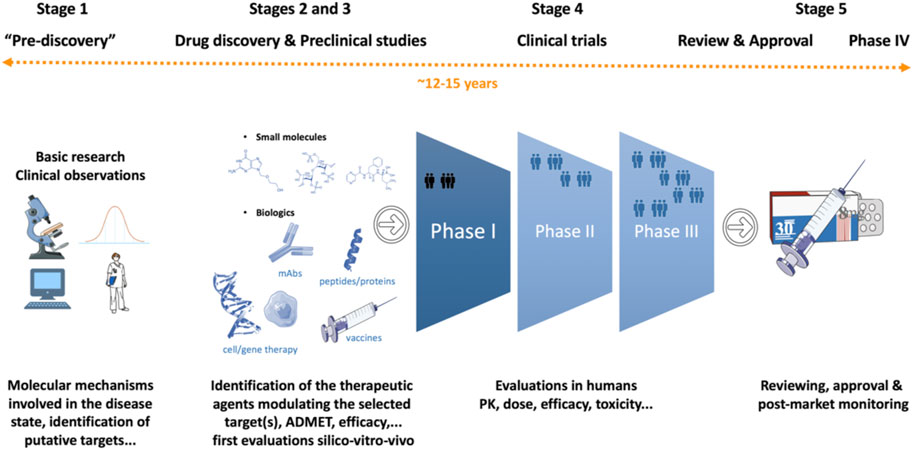
Frontiers Drug discovery and development: introduction to the

Preclinical Working Group National Institutes of Health (NIH)
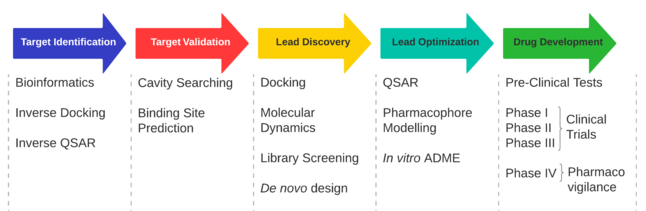
Drug discovery - Wikipedia

Quantum computing in drug development
Cells, Free Full-Text
Recomendado para você
-
brain test 196|TikTok Search15 abril 2025
-
 Brain Test Level 411 (NEW) Light up the birthday candles Answer15 abril 2025
Brain Test Level 411 (NEW) Light up the birthday candles Answer15 abril 2025 -
 brain test nível 41115 abril 2025
brain test nível 41115 abril 2025 -
 MicroRNA-411 and Its 5′-IsomiR Have Distinct Targets and Functions15 abril 2025
MicroRNA-411 and Its 5′-IsomiR Have Distinct Targets and Functions15 abril 2025 -
 The 411 on A1C: Normal A1C levels and 15 ways to lower high A1C15 abril 2025
The 411 on A1C: Normal A1C levels and 15 ways to lower high A1C15 abril 2025 -
Hashimoto's 411 - If your iron is low, what is the best15 abril 2025
-
 Classification of autism spectrum disorder based on sample entropy15 abril 2025
Classification of autism spectrum disorder based on sample entropy15 abril 2025 -
 Love Pins的LEVEL 411怎样通关?-百度经验15 abril 2025
Love Pins的LEVEL 411怎样通关?-百度经验15 abril 2025 -
 Studies add details about the brain, clues for future treatments15 abril 2025
Studies add details about the brain, clues for future treatments15 abril 2025 -
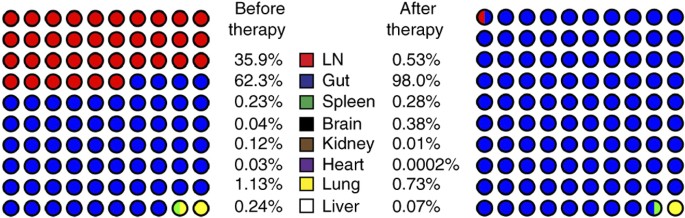 Defining total-body AIDS-virus burden with implications for15 abril 2025
Defining total-body AIDS-virus burden with implications for15 abril 2025
você pode gostar
-
 Stream Mangle FNAF Listen to Glitch Trap playlist online for15 abril 2025
Stream Mangle FNAF Listen to Glitch Trap playlist online for15 abril 2025 -
 DOOMED MEGALOPOLIS: THE LAST MEGALOPOLIS On Blu-ray From Media15 abril 2025
DOOMED MEGALOPOLIS: THE LAST MEGALOPOLIS On Blu-ray From Media15 abril 2025 -
 Nanatsu no Taizai: Imashime no Fukkatsu - Dublado - The Seven15 abril 2025
Nanatsu no Taizai: Imashime no Fukkatsu - Dublado - The Seven15 abril 2025 -
 Brasil perde nos pênaltis e é eliminado da Copa do Mundo do Catar15 abril 2025
Brasil perde nos pênaltis e é eliminado da Copa do Mundo do Catar15 abril 2025 -
 The Best iOS Games Of 2022 According To Metacritic - GameSpot15 abril 2025
The Best iOS Games Of 2022 According To Metacritic - GameSpot15 abril 2025 -
Influenciador que teria causado morte de adolescente ao dar 'grau' em moto volta a postar manobras - Notícias - R7 São Paulo15 abril 2025
-
UNCHARTED™: Legacy of Thieves Collection - PS515 abril 2025
-
 Floppa Cube - Today Was A Good Day (One color), Flop Flop Happy Floppa Friday, Racist War Crime Fun Tax Fraud15 abril 2025
Floppa Cube - Today Was A Good Day (One color), Flop Flop Happy Floppa Friday, Racist War Crime Fun Tax Fraud15 abril 2025 -
 O mundo todo deve ouvir o Evangelho para Jesus voltar? Veja o15 abril 2025
O mundo todo deve ouvir o Evangelho para Jesus voltar? Veja o15 abril 2025 -
 Fidel Castro · George Washington's Mount Vernon15 abril 2025
Fidel Castro · George Washington's Mount Vernon15 abril 2025



