FDA's Fast-Track for Rexulti Raises Concerns
Por um escritor misterioso
Last updated 15 abril 2025

CMS efforts to reduce use of unnecessary antipsychotics in nursing homes may conflict with marketing efforts for the drug.

FDA rushes approval of dementia drug that quadruples risk of death

FDA's accelerated drug approvals often lack confirmatory evidence : Shots - Health News : NPR
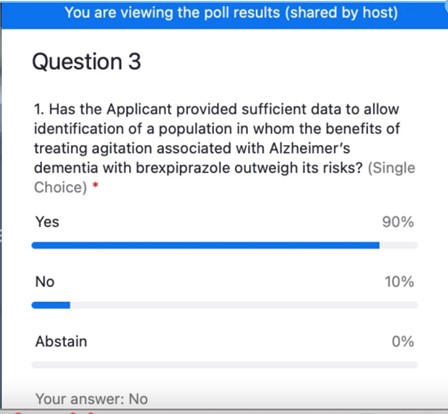
Not Everyone Agreed With FDA Approval of Antipsychotic Rexulti for Agitation - Mad In America

Not Everyone Agreed With FDA Approval of Antipsychotic Rexulti for Agitation - Mad In America

FDA-Approved Drugs to Treat Schizophrenia Journal of Psychosocial Nursing and Mental Health Services

FDA Approves First Treatment for Alzheimer's Agitation
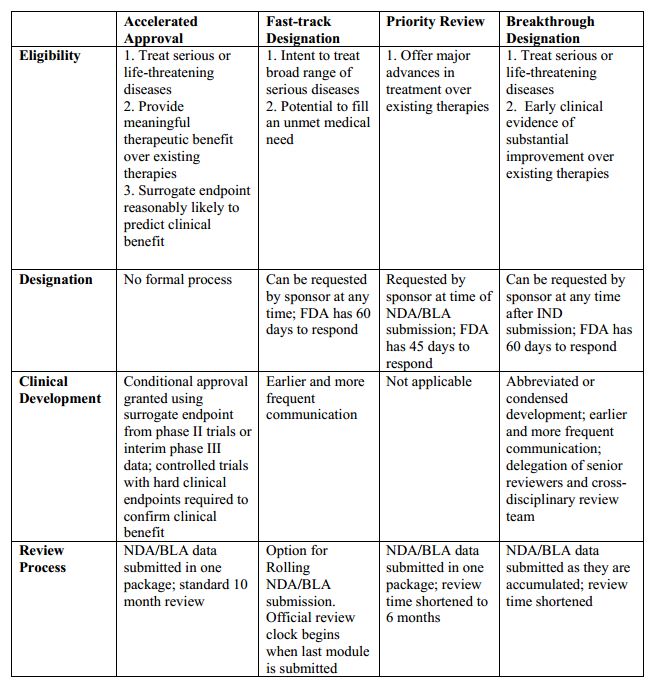
FDA Expedited Review Programs - Friends of Cancer Research

FDA approves supplemental new drug application for Rexulti to treat Alzheimer's agitation
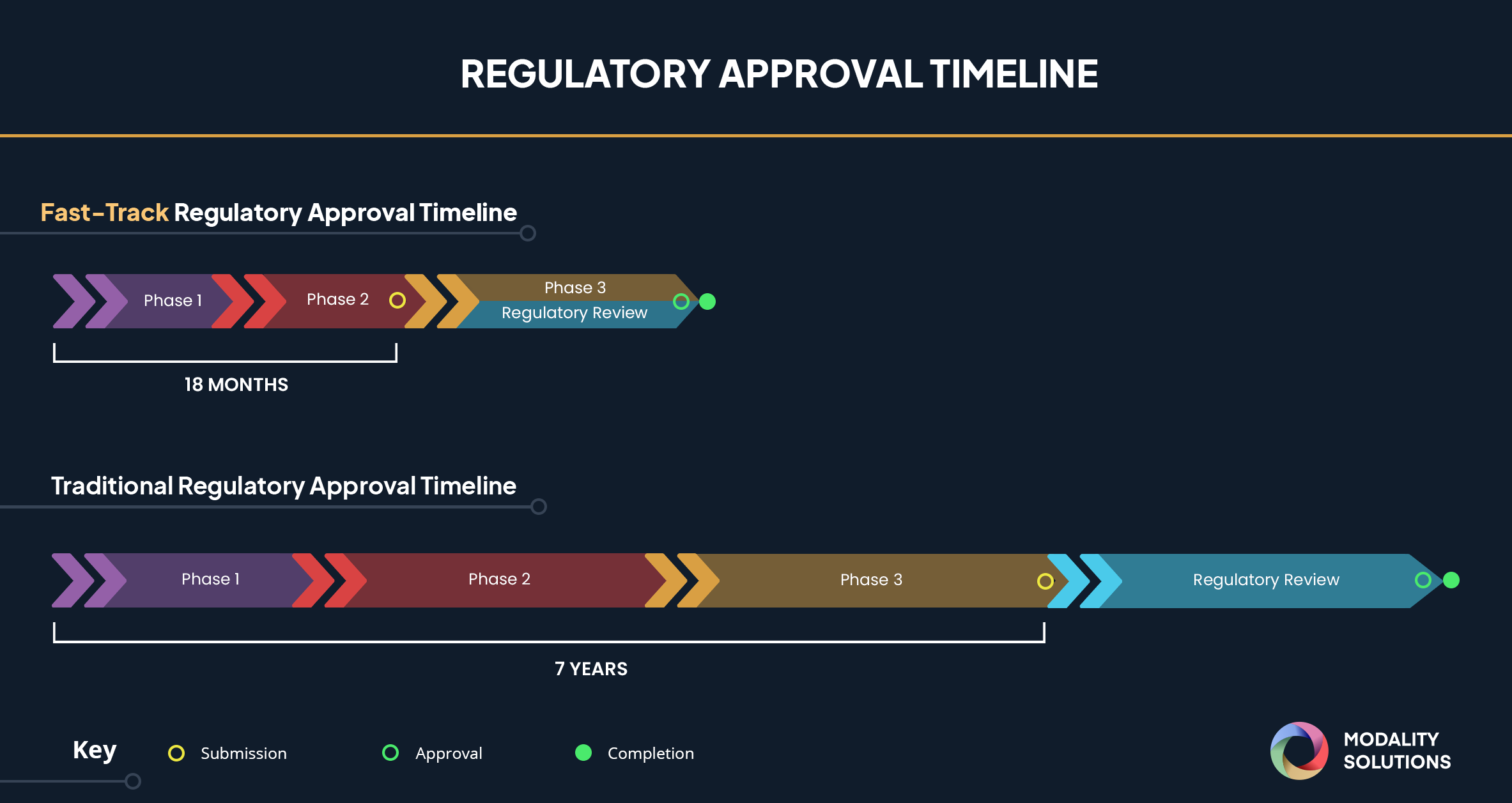
FDA's Fast Track Approval Coronavirus Treatment Acceleration Program
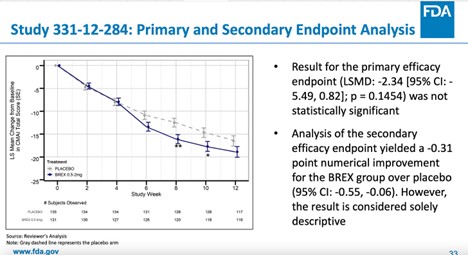
Not Everyone Agreed With FDA Approval of Antipsychotic Rexulti for Agitation - Mad In America

FDA fast-tracking approval of new drugs — fewer trials, less info
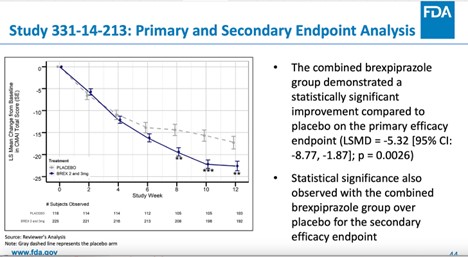
Not Everyone Agreed With FDA Approval of Antipsychotic Rexulti for Agitation - Mad In America
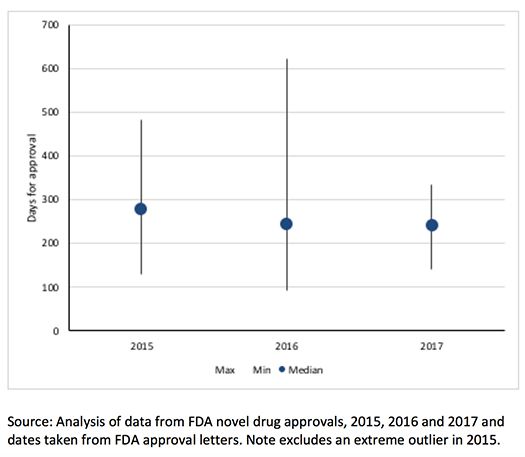
How Fast are the FDA Fast Lanes?

FDA Fast Track Designation for Ad-RTS-hIL-12 plus Veledimex for Recurrent Glioblastoma

How the FDA approved an antipsychotic that failed to show a meaningful benefit but raised the risk of death
Recomendado para você
-
 Otsuka America Pharmaceutical 59148003613 - McKesson Medical-Surgical15 abril 2025
Otsuka America Pharmaceutical 59148003613 - McKesson Medical-Surgical15 abril 2025 -
 Order Rexulti 2 mg with free shipping - Online Canadian Pharmacy15 abril 2025
Order Rexulti 2 mg with free shipping - Online Canadian Pharmacy15 abril 2025 -
 Buy Rexulti Online – Brexpiprazole Canada15 abril 2025
Buy Rexulti Online – Brexpiprazole Canada15 abril 2025 -
Brexpiprazole (Rexulti): Uses, Side Effects, Warnings & More - GoodRx15 abril 2025
-
 REXULTI TV Spot, 'Isolated'15 abril 2025
REXULTI TV Spot, 'Isolated'15 abril 2025 -
 Rexulti, the first drug to relieve Alzheimer's emotions - TimesKuwait15 abril 2025
Rexulti, the first drug to relieve Alzheimer's emotions - TimesKuwait15 abril 2025 -
 REXULTI® (brexpiprazole) agitation that may happen with dementia due to Alzheimer's disease15 abril 2025
REXULTI® (brexpiprazole) agitation that may happen with dementia due to Alzheimer's disease15 abril 2025 -
 Rexulti Advertisement Poster for Sale by BLTC15 abril 2025
Rexulti Advertisement Poster for Sale by BLTC15 abril 2025 -
 Pharmaceutical Business Products15 abril 2025
Pharmaceutical Business Products15 abril 2025 -
 REXULTI 0,5MG CAIXA COM 30 COMPRIMIDOS REVESTIDOS (C1) - Farmácias CallFarma15 abril 2025
REXULTI 0,5MG CAIXA COM 30 COMPRIMIDOS REVESTIDOS (C1) - Farmácias CallFarma15 abril 2025
você pode gostar
-
 11 Sarcastic & Funny Last-Minute Holiday Gifts — $25 or15 abril 2025
11 Sarcastic & Funny Last-Minute Holiday Gifts — $25 or15 abril 2025 -
 Leifon Alseif - Chrome Shelled Regios - Zerochan Anime Image Board15 abril 2025
Leifon Alseif - Chrome Shelled Regios - Zerochan Anime Image Board15 abril 2025 -
 Pokemon 151 Zapdos SAR, Hobbies & Toys, Toys & Games on Carousell15 abril 2025
Pokemon 151 Zapdos SAR, Hobbies & Toys, Toys & Games on Carousell15 abril 2025 -
 It's a day late, but here are some Juggernaut (Zombie) Treats! : r/ MarvelStrikeForce15 abril 2025
It's a day late, but here are some Juggernaut (Zombie) Treats! : r/ MarvelStrikeForce15 abril 2025 -
 Pin by ff._destroy on t shots para roblox, Free tshirt, Roblox t shirts, Free t shirt design15 abril 2025
Pin by ff._destroy on t shots para roblox, Free tshirt, Roblox t shirts, Free t shirt design15 abril 2025 -
 Marrocos 0 - 0 Espanha (3-0, G.P) (Relato) Oitavos de Final do Mundial 2022 - A Primeira Rádio Desporto - Golo FM15 abril 2025
Marrocos 0 - 0 Espanha (3-0, G.P) (Relato) Oitavos de Final do Mundial 2022 - A Primeira Rádio Desporto - Golo FM15 abril 2025 -
Dunder Mifflin Conference Room15 abril 2025
-
 Clash of Kings UK15 abril 2025
Clash of Kings UK15 abril 2025 -
Pra desespero dos haters, o 300º episódio de Os Jovens Titãs em Ação estreia essa semana no Cartoon Network - TVLaint Brasil15 abril 2025
-
 Play Fortnite with Xbox Cloud Gaming For Free Without any15 abril 2025
Play Fortnite with Xbox Cloud Gaming For Free Without any15 abril 2025

