What Does the IRB Review?, Research
Por um escritor misterioso
Last updated 15 abril 2025

Below are the elements the IRB looks for when reviewing research. Federal regulations 45 CFR 46.111 and 21 CFR 56.111 outline the requirements for approval of non-exempt human subjects research. To obtain IRB approval, the IRB must have enough information to determine the criteria in each of the sections below are satisfied.
Master's Students and the IRB, 2021, IRB Blog, Institutional Review Board

Human Subject Participation - Office of Research Integrity
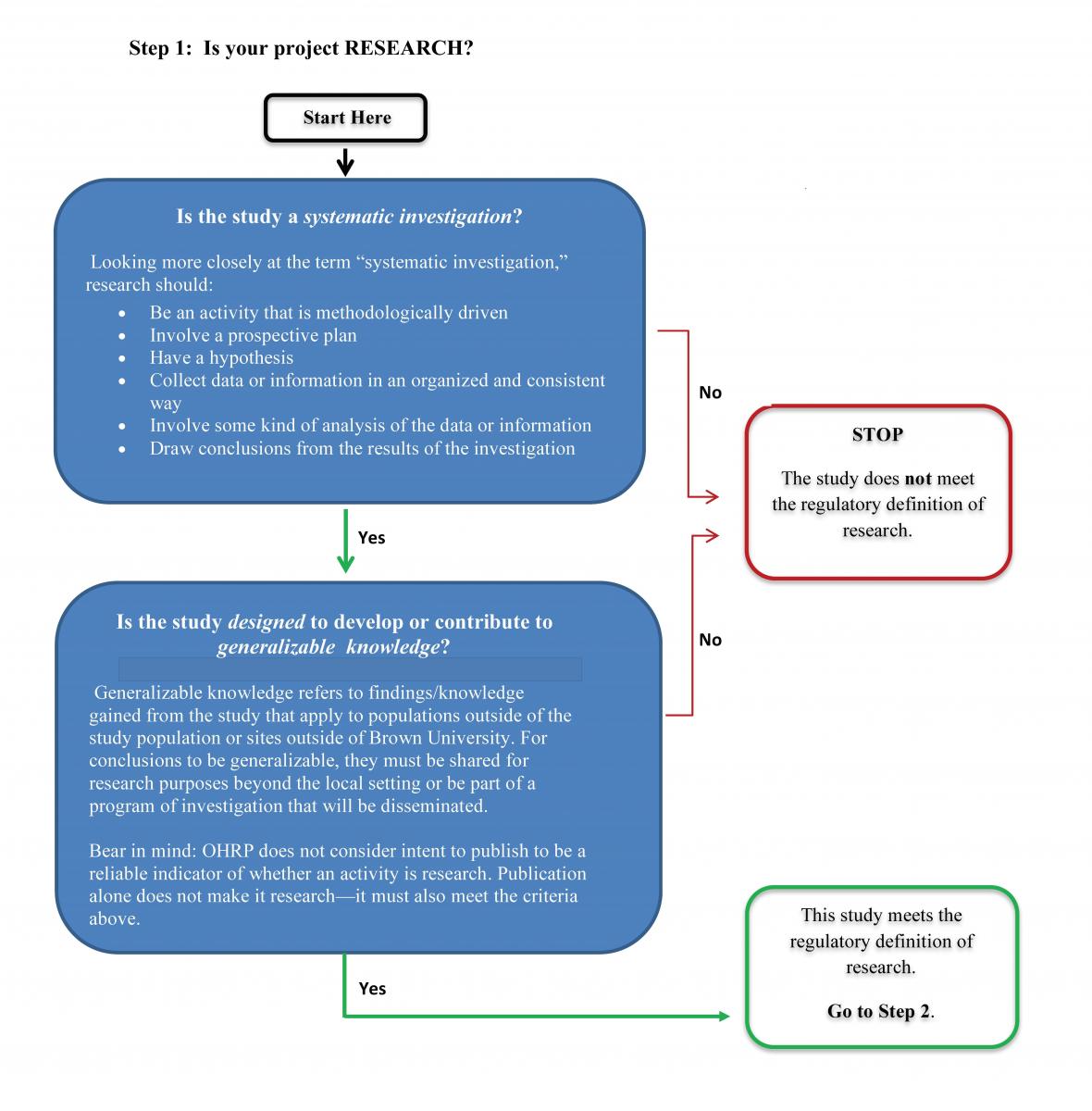
Does My Project Need IRB Review?, Research at Brown
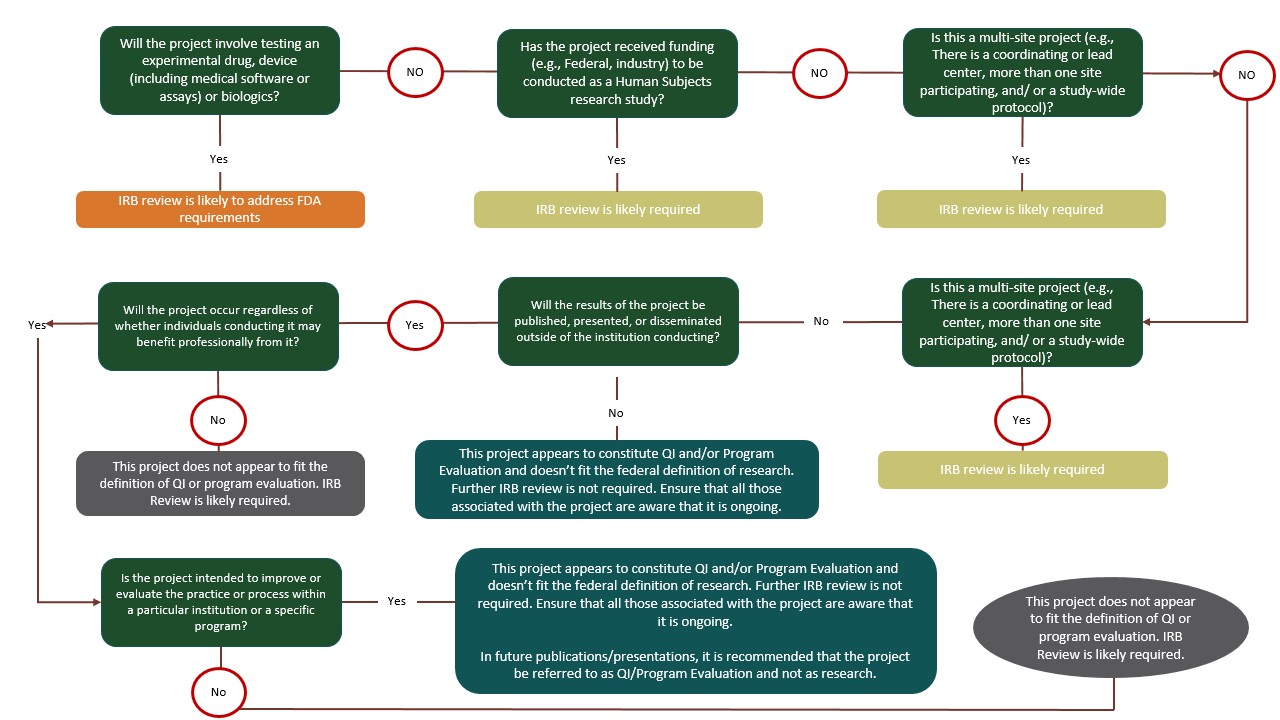
FAQs - Vice President For Research

New Investigators - Investigators - Institutional Review Board - Campbell University

Levels of IRB Review
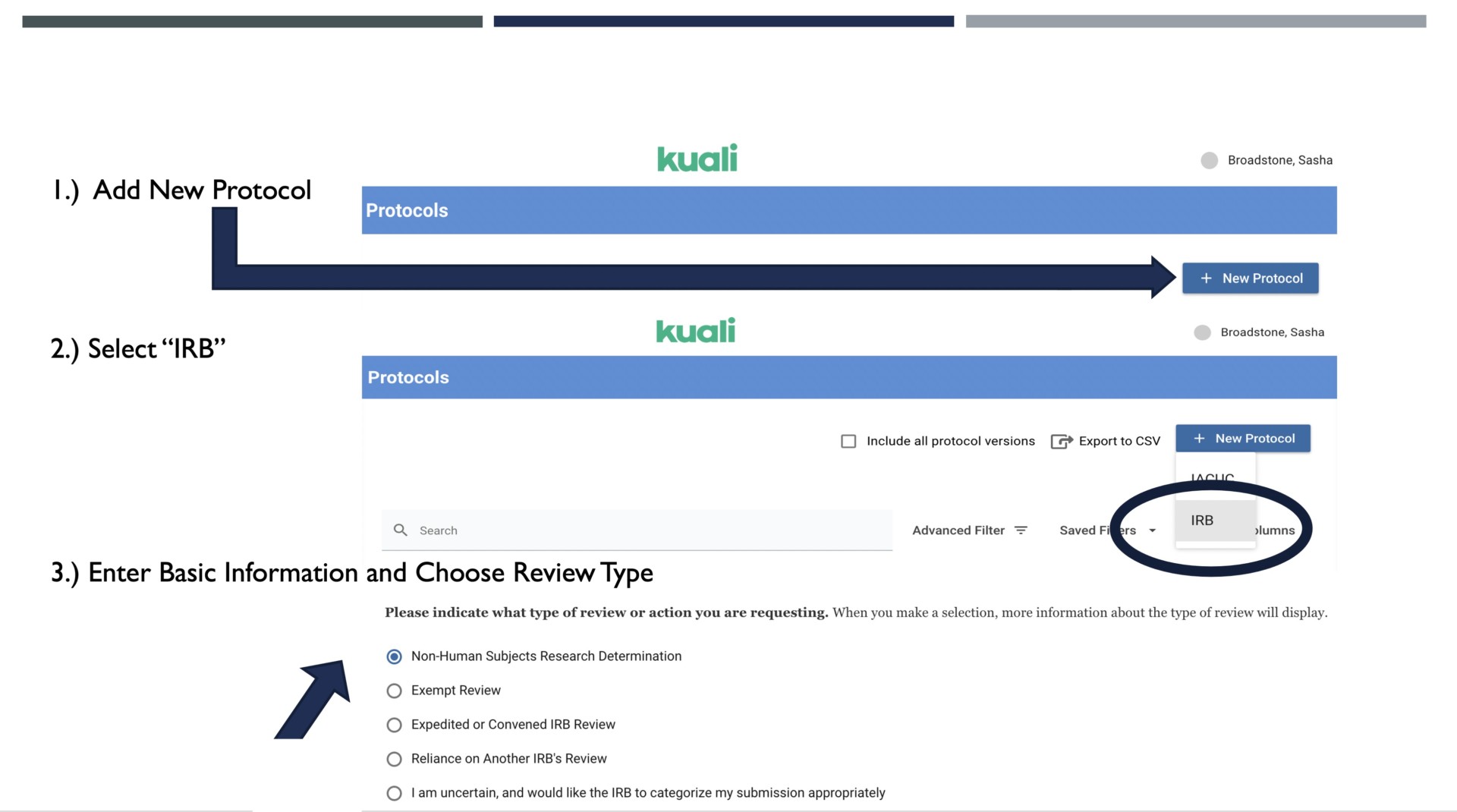
Do I Need IRB Review?, Institutional Review Board
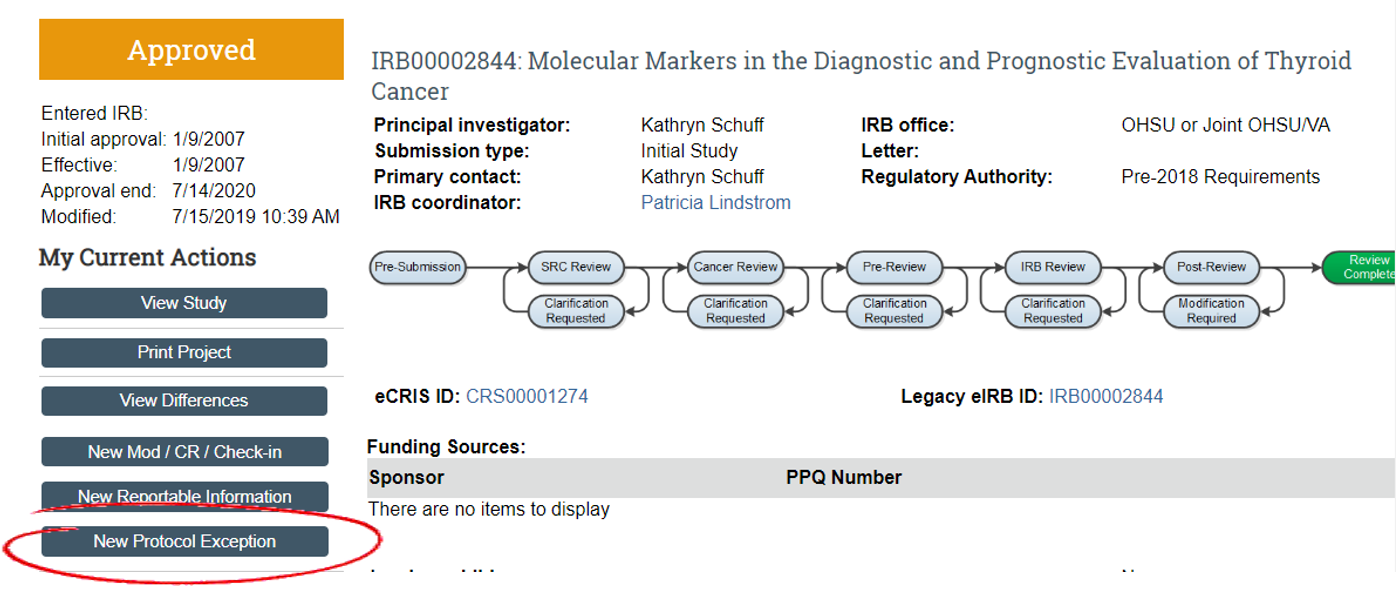
Institutional Review Board (IRB)
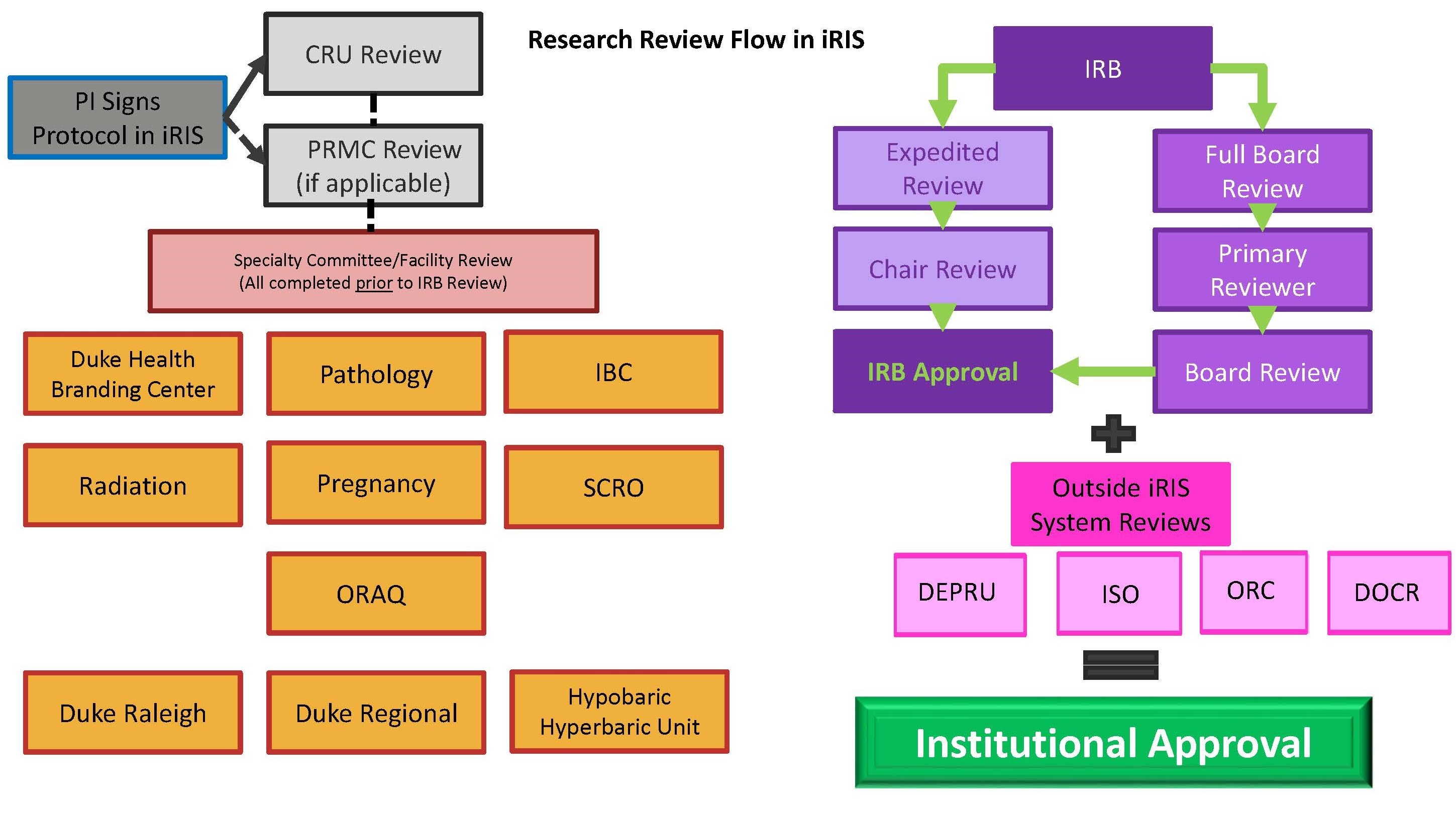
IRB Process Duke Health Institutional Review Board

Requirements for Institutional Review Board (IRB) Review and HIPAA Waiver Documentation for RIF DUA Request Submissions

IRB Performance & Metrics
Get to Know a Review Category - Exempt Category 4, 2021, IRB Blog, Institutional Review Board
Recomendado para você
-
Instituto Rio Branco15 abril 2025
-
 Campanha em benefício do Instituto de Oncologia – IRBSL15 abril 2025
Campanha em benefício do Instituto de Oncologia – IRBSL15 abril 2025 -
Christian Martinez on Instagram: May is my favorite month of the year for three reasons, now four. Thanks for always helping me pick up the pieces💚15 abril 2025
-
 Impressos Portão15 abril 2025
Impressos Portão15 abril 2025 -
 Irbsl (irbsl9201) - Profile15 abril 2025
Irbsl (irbsl9201) - Profile15 abril 2025 -
 适用于丰田本田日产车载腰靠座椅头颈枕抱枕四件套空调被logo定制-Taobao15 abril 2025
适用于丰田本田日产车载腰靠座椅头颈枕抱枕四件套空调被logo定制-Taobao15 abril 2025 -
 Memgift-Pulsera-de-piña-personalizada-para-mujer-Regalos-ins15 abril 2025
Memgift-Pulsera-de-piña-personalizada-para-mujer-Regalos-ins15 abril 2025 -
 eProtocol - SLU - Protocol Management System15 abril 2025
eProtocol - SLU - Protocol Management System15 abril 2025 -
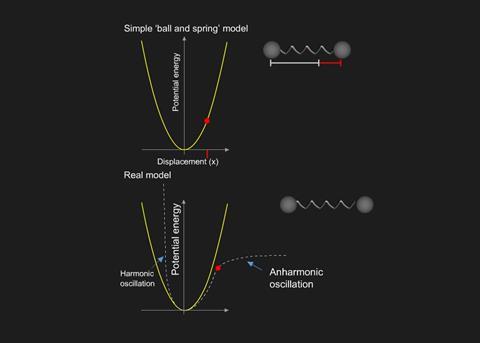 Infrared (IR) spectroscopy: Energy levels, Resource15 abril 2025
Infrared (IR) spectroscopy: Energy levels, Resource15 abril 2025 -
 Global recognition – RSB15 abril 2025
Global recognition – RSB15 abril 2025
você pode gostar
-
 Papa's Bakeria15 abril 2025
Papa's Bakeria15 abril 2025 -
 Suzunari First/Uniform, World Trigger Wiki15 abril 2025
Suzunari First/Uniform, World Trigger Wiki15 abril 2025 -
 New Art from Crunchyroll for the Anime, featuring the Hokages and Team 7! : r/Naruto15 abril 2025
New Art from Crunchyroll for the Anime, featuring the Hokages and Team 7! : r/Naruto15 abril 2025 -
 How to Draw Anime for Beginners15 abril 2025
How to Draw Anime for Beginners15 abril 2025 -
 INTRUDER Mandela Catalogue Song (Original) by longestsoloever15 abril 2025
INTRUDER Mandela Catalogue Song (Original) by longestsoloever15 abril 2025 -
 Yaharong - Liquipedia League of Legends Wiki15 abril 2025
Yaharong - Liquipedia League of Legends Wiki15 abril 2025 -
 Hyakuren no Haou to Seiyaku no Valkyria Folder by Edgina36 on DeviantArt15 abril 2025
Hyakuren no Haou to Seiyaku no Valkyria Folder by Edgina36 on DeviantArt15 abril 2025 -
Tabela Fipe Brasil – Apps bei Google Play15 abril 2025
-
 The Art of Feng Shui in 2023 Feng shui, Feng shui items, Fung shui15 abril 2025
The Art of Feng Shui in 2023 Feng shui, Feng shui items, Fung shui15 abril 2025 -
 Sopić nakon prvog poraza na Rujevici: “Igrači nisu zaslužili ovakve povike s tribina”15 abril 2025
Sopić nakon prvog poraza na Rujevici: “Igrači nisu zaslužili ovakve povike s tribina”15 abril 2025

