What You Should Know About CSV in Pharma
Por um escritor misterioso
Last updated 15 abril 2025

Learn more about computer system validation, which is required by the FDA and other global regulatory bodies for drug and medical device manufacturers.

Pharmaceutical Computer Validation Certificate Program • NACPT

Software Validation: Here's How We Do It - Apprentice

Why is Computer System Validation so important? - Express Pharma

Software Validation: Here's How We Do It - Apprentice
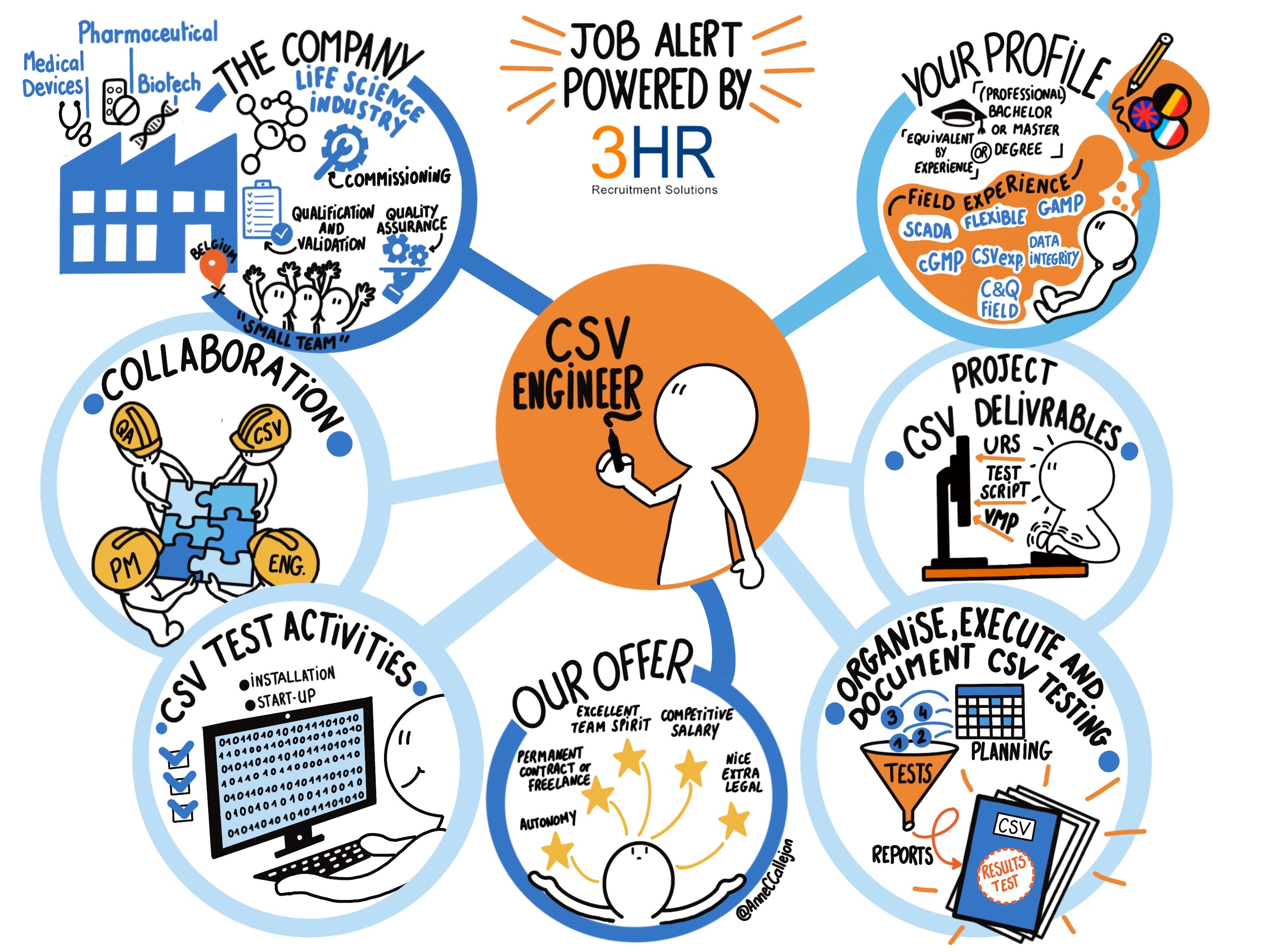
Candidate, find the job, submit CV or mission request - 3HR

What Is a CSV Engineer & What Do They Do?

Computer System Validation: 7 Best Practices That Should Be Followed
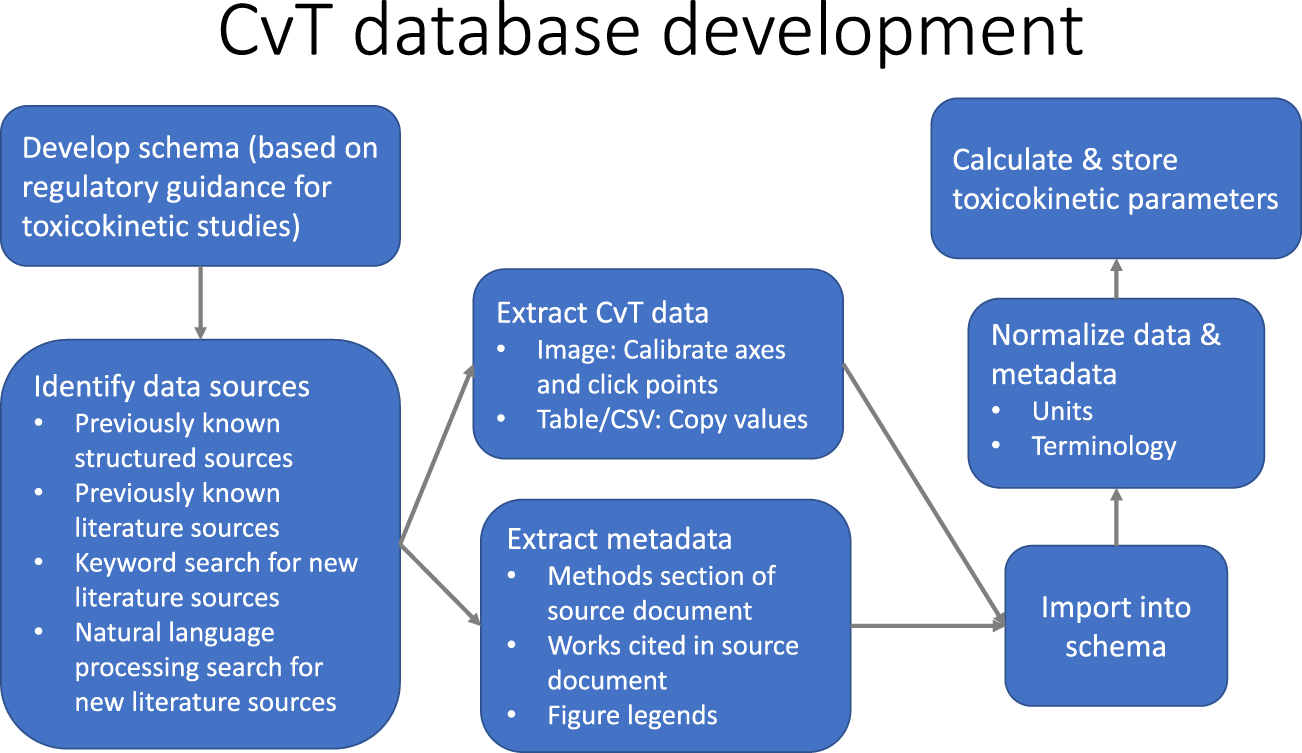
Database of pharmacokinetic time-series data and parameters for 144 environmental chemicals

PDF) Computer system validation in the perspective of the pharmaceutical industry

LIMS Validation Plan

What does a CSV Engineer do? // Talentmark

9 of the Best Online CSV Resources for Pharma Validation Professionals
Risk-based approach driving manufacturing improvements
Recomendado para você
-
 COLÉGIO SÃO VICENTE DE PAULO SÃO LUÍS / MA15 abril 2025
COLÉGIO SÃO VICENTE DE PAULO SÃO LUÍS / MA15 abril 2025 -
CSVP - MARANhHÃO15 abril 2025
-
 Save on Columbus Salame Sopressata Chub Order Online Delivery15 abril 2025
Save on Columbus Salame Sopressata Chub Order Online Delivery15 abril 2025 -
 Pacific Merchant Shipping Association15 abril 2025
Pacific Merchant Shipping Association15 abril 2025 -
 ASCM San Fernando Valley Chapter CPIM CSCP CLTD15 abril 2025
ASCM San Fernando Valley Chapter CPIM CSCP CLTD15 abril 2025 -
 Creating a csv file in other format than comma for master or15 abril 2025
Creating a csv file in other format than comma for master or15 abril 2025 -
CSVPA Cambridge School of Visual & Performing Arts15 abril 2025
-
 Colégio São Vicente de Paulo15 abril 2025
Colégio São Vicente de Paulo15 abril 2025 -
 2017 Audi A3 2.0 TDI S LINE 2.0 Diesel Manual - £15250 - PMA Cars15 abril 2025
2017 Audi A3 2.0 TDI S LINE 2.0 Diesel Manual - £15250 - PMA Cars15 abril 2025 -
 Computer System Validation (CSV) to Computer Software Assurance (CSA): Taking a More Risked-Based Approach - Verista15 abril 2025
Computer System Validation (CSV) to Computer Software Assurance (CSA): Taking a More Risked-Based Approach - Verista15 abril 2025
você pode gostar
-
 Mod The Sims - When I Grow Up Play Outfits15 abril 2025
Mod The Sims - When I Grow Up Play Outfits15 abril 2025 -
 Best chess players never to become World Champion - part one - Viktor Korchnoi - Chessentials15 abril 2025
Best chess players never to become World Champion - part one - Viktor Korchnoi - Chessentials15 abril 2025 -
 Tatuagem Alien Tattoo, O novo Site do Micael Tattoo Studio …15 abril 2025
Tatuagem Alien Tattoo, O novo Site do Micael Tattoo Studio …15 abril 2025 -
📕 Sono bisque doll - TV/Anime Artbook 😍 . . 🟦 Tags: #mangacollectio15 abril 2025
-
 Stranger Things: elenco comenta sexualidade de Will; confira!15 abril 2025
Stranger Things: elenco comenta sexualidade de Will; confira!15 abril 2025 -
 Ink Sans vs Cross Sans ANIMATION15 abril 2025
Ink Sans vs Cross Sans ANIMATION15 abril 2025 -
 Champion (2018) 챔피언 Review15 abril 2025
Champion (2018) 챔피언 Review15 abril 2025 -
 Identifying Birds of Prey 215 abril 2025
Identifying Birds of Prey 215 abril 2025 -
 The Cabaret South Beach - All You Need to Know BEFORE You Go (with Photos)15 abril 2025
The Cabaret South Beach - All You Need to Know BEFORE You Go (with Photos)15 abril 2025 -
 Fast & Furious Tokyo Drift 350Z For Sale15 abril 2025
Fast & Furious Tokyo Drift 350Z For Sale15 abril 2025


