Molecular signatures of inherited and acquired sporadic late onset
Por um escritor misterioso
Last updated 14 abril 2025
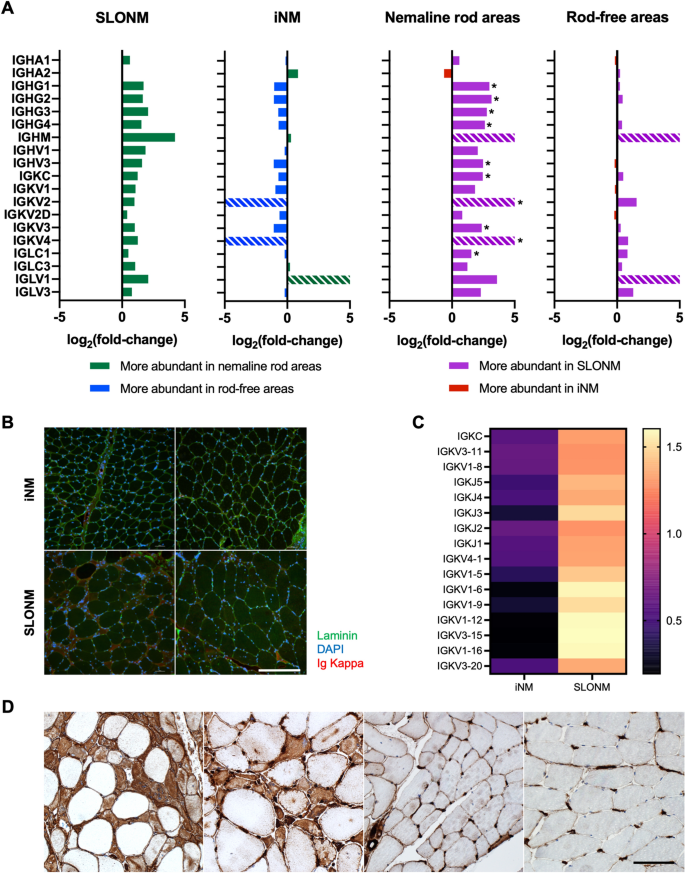
Acquired sporadic late onset nemaline myopathy (SLONM) and inherited nemaline myopathy (iNM) both feature accumulation of nemaline rods in muscle fibers. Unlike iNM, SLONM is amenable to therapy. The distinction between these disorders is therefore crucial when the diagnosis remains ambiguous after initial investigations. We sought to identify biomarkers facilitating this distinction and to investigate the pathophysiology of nemaline rod formation in these different disorders. Twenty-two muscle samples from patients affected by SLONM or iNM underwent quantitative histological analysis, laser capture microdissection for proteomic analysis of nemaline rod areas and rod-free areas, and transcriptomic analysis. In all iNM samples, nemaline rods were found in subsarcolemmal or central aggregates, whereas they were diffusely distributed within muscle fibers in most SLONM samples. In SLONM, muscle fibers harboring nemaline rods were smaller than those without rods. Necrotic fibers, increased endomysial connective tissue, and atrophic fibers filled with nemaline rods were more common in SLONM. Proteomic analysis detected differentially expressed proteins between nemaline rod areas and rod-free areas, as well as between SLONM and iNM. These differentially expressed proteins implicated immune, structural, metabolic, and cellular processes in disease pathophysiology. Notably, immunoglobulin overexpression with accumulation in nemaline rod areas was detected in SLONM. Transcriptomic analysis corroborated proteomic findings and further revealed substantial gene expression differences between SLONM and iNM. Overall, we identified unique pathological and molecular signatures associated with SLONM and iNM, suggesting distinct underlying pathophysiological mechanisms. These findings represent a step towards enhanced diagnostic tools and towards development of treatments for SLONM.

Molecular mechanisms underlying totipotency
IJMS, Free Full-Text

Molecular profiling in breast cancer

Proteomics of brain, CSF, and plasma identifies molecular

Functional genomics, genetic risk profiling and cell phenotypes in

A pattern-based approach to the interpretation of skeletal muscle

Disruption of Fgf13 Causes Synaptic Excitatory–Inhibitory

Cardiomyocyte Maturation

Bone marrow-derived inducible microglia-like cells ameliorate

Proteomics of brain, CSF, and plasma identifies molecular

Mitigating age-related somatic mutation burden: Trends in

Single-Cell Epigenomics and Functional Fine-Mapping of

Clinicopathologic Profiles of Sporadic Late-Onset Nemaline

Clinicopathologic Profiles of Sporadic Late-Onset Nemaline
Recomendado para você
-
 The systemic anti-microbiota IgG repertoire can identify gut14 abril 2025
The systemic anti-microbiota IgG repertoire can identify gut14 abril 2025 -
 Fetal genome profiling at 5 weeks of gestation after noninvasive14 abril 2025
Fetal genome profiling at 5 weeks of gestation after noninvasive14 abril 2025 -
 Biology, Free Full-Text14 abril 2025
Biology, Free Full-Text14 abril 2025 -
 Altered Fc galactosylation in IgG4 is a potential serum marker for14 abril 2025
Altered Fc galactosylation in IgG4 is a potential serum marker for14 abril 2025 -
 Transcriptional and clonal characterization of B cell plasmablast14 abril 2025
Transcriptional and clonal characterization of B cell plasmablast14 abril 2025 -
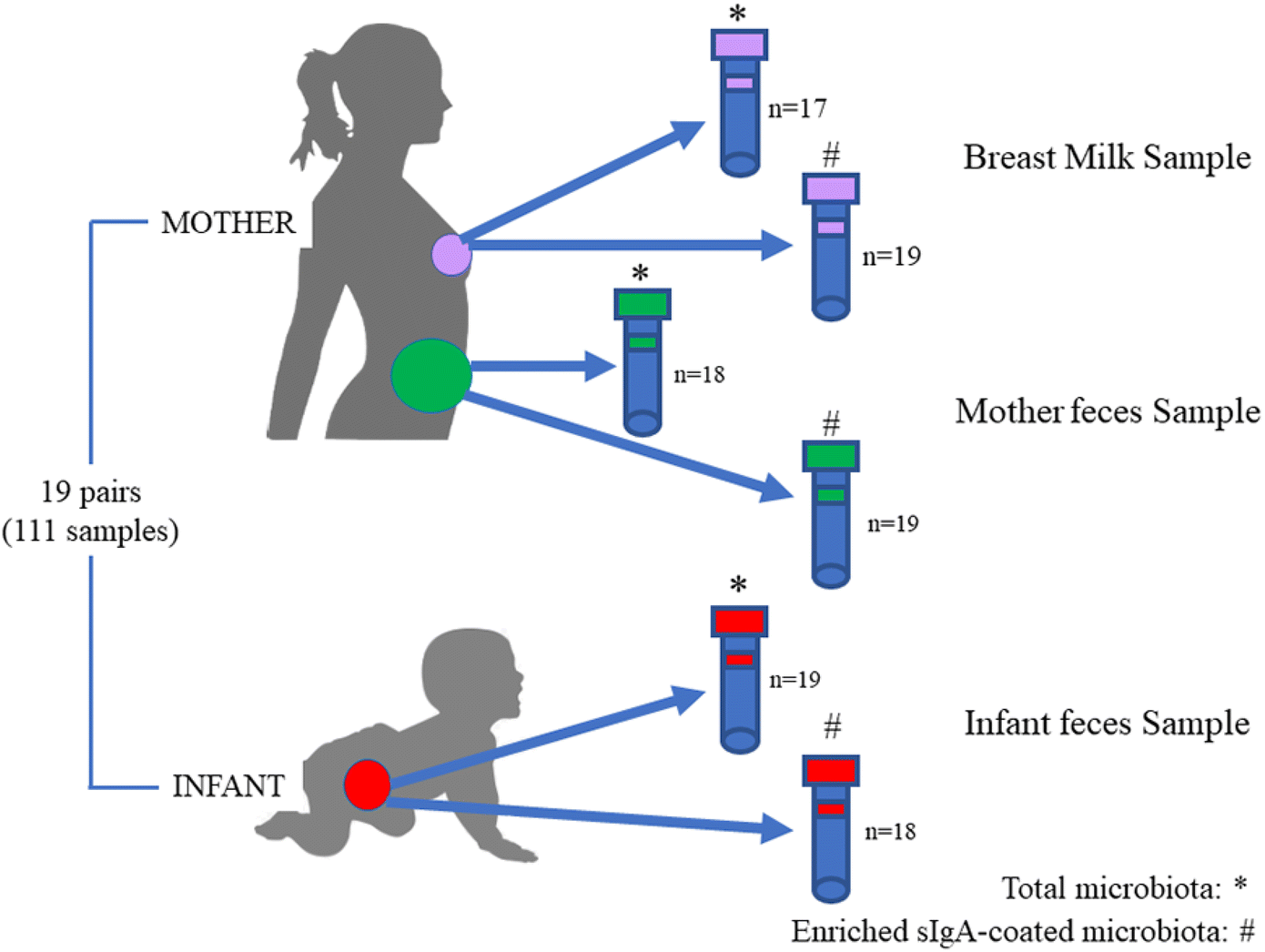 Widespread vertical transmission of secretory immunoglobulin A14 abril 2025
Widespread vertical transmission of secretory immunoglobulin A14 abril 2025 -
 The systemic anti-microbiota IgG repertoire can identify gut14 abril 2025
The systemic anti-microbiota IgG repertoire can identify gut14 abril 2025 -
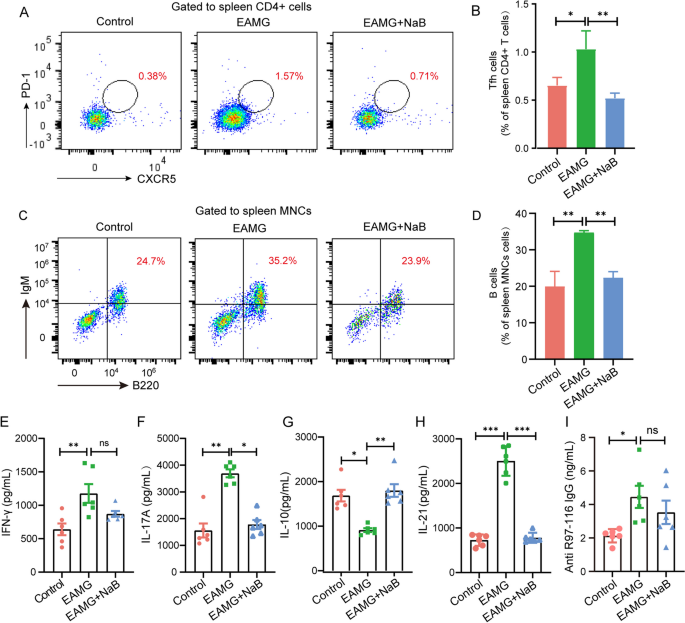 Sodium butyrate alleviates R97-116 peptide-induced myasthenia14 abril 2025
Sodium butyrate alleviates R97-116 peptide-induced myasthenia14 abril 2025 -
 Full article: Impact of IgG subclass on monoclonal antibody14 abril 2025
Full article: Impact of IgG subclass on monoclonal antibody14 abril 2025 -
Michael Ramova (@ramova) / X14 abril 2025
você pode gostar
-
 Read Tokyo Revengers Manga Chapter 264 in English Free Online14 abril 2025
Read Tokyo Revengers Manga Chapter 264 in English Free Online14 abril 2025 -
TC Ensina: como cancelar sua assinatura na Netflix pelo celular ou computador14 abril 2025
-
 Digitized) BFDI - TB x GB (Club Penguin version) by CadenFeather14 abril 2025
Digitized) BFDI - TB x GB (Club Penguin version) by CadenFeather14 abril 2025 -
 Notícias, Colégio Bom Jesus, Alunos do Bom Jesus de Blumenau são destaque em competições de Xadrez14 abril 2025
Notícias, Colégio Bom Jesus, Alunos do Bom Jesus de Blumenau são destaque em competições de Xadrez14 abril 2025 -
 Análise técnica de Quake 2 2023: é assim que se remasteriza um jogo14 abril 2025
Análise técnica de Quake 2 2023: é assim que se remasteriza um jogo14 abril 2025 -
 2,289 Videogame Drawing Images, Stock Photos, 3D objects, & Vectors14 abril 2025
2,289 Videogame Drawing Images, Stock Photos, 3D objects, & Vectors14 abril 2025 -
 Ariana Grande CONFIRMS Relationship In 'Stuck With U' Music Video14 abril 2025
Ariana Grande CONFIRMS Relationship In 'Stuck With U' Music Video14 abril 2025 -
 School Library Pokémon Club Application14 abril 2025
School Library Pokémon Club Application14 abril 2025 -
 When Grandmasters Blunder. A statistical analysis of chess.14 abril 2025
When Grandmasters Blunder. A statistical analysis of chess.14 abril 2025 -
 Mapas de Call of Duty Mobile Conheça os mapas mais icônicos de toda franquia14 abril 2025
Mapas de Call of Duty Mobile Conheça os mapas mais icônicos de toda franquia14 abril 2025
