ANANDA Scientific Announces FDA approval of the IND for the Clinical Trial on the Treatment of Opioid Use Disorder (OUD)
Por um escritor misterioso
Last updated 16 abril 2025

ANANDA Scientific Inc., (a biotech pharma company) today announced approval by the U.S. Food and Drug Administration (FDA) of the Investigational New
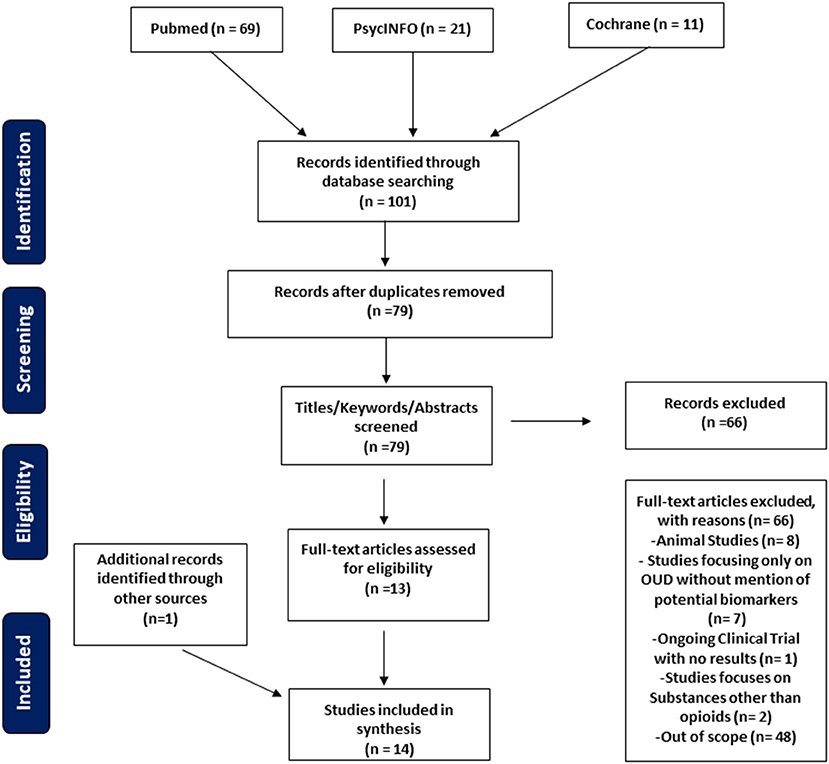
Frontiers A Systematic Review of Opioid Use Disorder and Related Biomarkers

ANANDA Scientific and David Geffen School of Medicine UCLA Announce Clinical Trial Utilizing Liquid StructureTM Cannabidiol (CBD) for the Treatment of Opioid Use Disorder (OUD)

A Decade of FDA-Approved Drugs (2010–2019): Trends and Future Directions

TESCREAL hallucinations: Psychedelic and AI hype as inequality engines in: Journal of Psychedelic StudiesOnline First

ANANDA Scientific

Nantheia ATL5: A New Hope for Beating Opioid Addiction - Treatment Magazine

The Opioid Crisis and Recent Federal Policy Responses
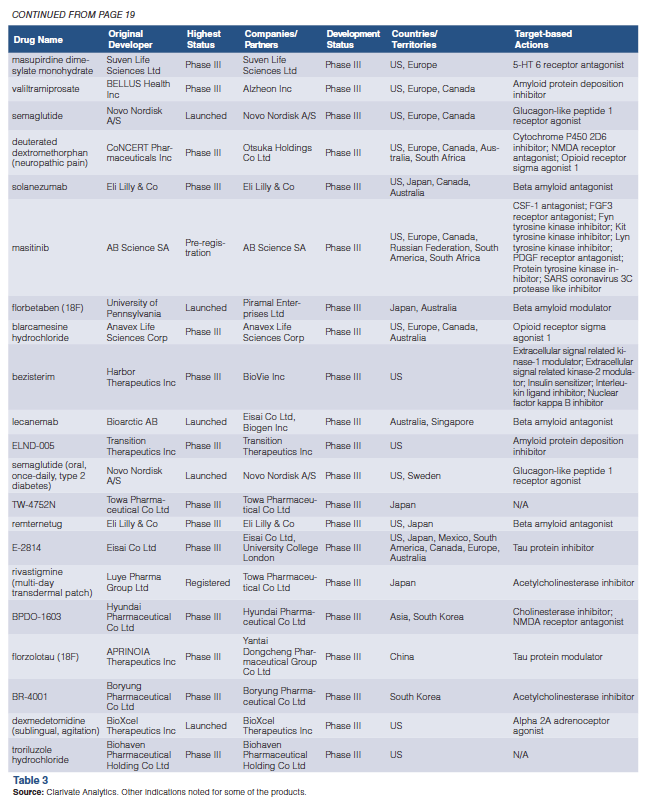
2024 Pipeline Report: First-World Focus

Jnana Therapeutics Announces FDA Clearance of IND Application for JNT-517 for the Treatment of Phenylketonuria

Neurological Archives - Page 2 of 21 - Drug Delivery Business

ANANDA Scientific Announces FDA approval of the IND for the Clinical Trial on the Treatment of Opioid Use Disorder (OUD)
Recomendado para você
-
 Ananda cosmetic16 abril 2025
Ananda cosmetic16 abril 2025 -
Ananda A Andrade - Business Owner - Click Midas Media16 abril 2025
-
 Ananda - Apple Music16 abril 2025
Ananda - Apple Music16 abril 2025 -
 ananda on X: ♥️ / X16 abril 2025
ananda on X: ♥️ / X16 abril 2025 -
Ananda - Indian Restaurant, Vegetarian, Kosher16 abril 2025
-
 HiFiMan Ananda Review16 abril 2025
HiFiMan Ananda Review16 abril 2025 -
 Ananda Los Angeles16 abril 2025
Ananda Los Angeles16 abril 2025 -
Ananda Mallawatantri - Advisor to Government - Presidential Secretariat16 abril 2025
-
 What is Ananda? Ananda Sacramento16 abril 2025
What is Ananda? Ananda Sacramento16 abril 2025 -
 Full Spectrum CBD, Pharmacy-Grade16 abril 2025
Full Spectrum CBD, Pharmacy-Grade16 abril 2025
você pode gostar
-
La Brasa Burger Lavras - Hamburgueria em Centro16 abril 2025
-
 NOVA ROUPA DE GRAÇA!? BALANCEAMENTO DE ARMAS, NOVA PARCERIA COM A16 abril 2025
NOVA ROUPA DE GRAÇA!? BALANCEAMENTO DE ARMAS, NOVA PARCERIA COM A16 abril 2025 -
 Create a Sans Au's-Always Update Tier List - TierMaker16 abril 2025
Create a Sans Au's-Always Update Tier List - TierMaker16 abril 2025 -
 Tata Steel Chess Masters 2023 - Toda la información16 abril 2025
Tata Steel Chess Masters 2023 - Toda la información16 abril 2025 -
 Assinantes EA Play e Xbox Game Pass Ultimate terão acesso ilimitado a Knockout City no lançamento - Gamer Point16 abril 2025
Assinantes EA Play e Xbox Game Pass Ultimate terão acesso ilimitado a Knockout City no lançamento - Gamer Point16 abril 2025 -
 169 Macaco Aranha Fotos, Imagens e Fundo para Download Gratuito - Pngtree16 abril 2025
169 Macaco Aranha Fotos, Imagens e Fundo para Download Gratuito - Pngtree16 abril 2025 -
 Queen Bee - The Award Winning Family Game by Mike P Bruner — Kickstarter16 abril 2025
Queen Bee - The Award Winning Family Game by Mike P Bruner — Kickstarter16 abril 2025 -
Gamers - Mafia-3 (full version +crack) 100% Size- 40gb16 abril 2025
-
 Gibson records releases Slash new album, 4 – t.blog16 abril 2025
Gibson records releases Slash new album, 4 – t.blog16 abril 2025 -
 Why GymTok Believes Baki is the Greatest Anime Character - Anime Herald16 abril 2025
Why GymTok Believes Baki is the Greatest Anime Character - Anime Herald16 abril 2025



