hydrogen orbital wavefunction
Por um escritor misterioso
Last updated 26 abril 2025
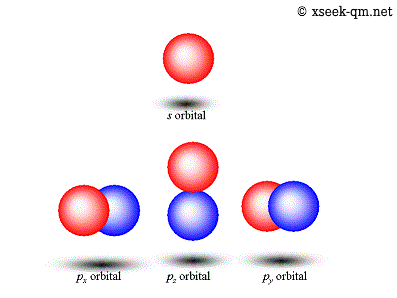
Atomic Orbital

Hydrogen atom - Wikipedia
The wave function of quantum mechanics - Mysterious World of Quantum Theory
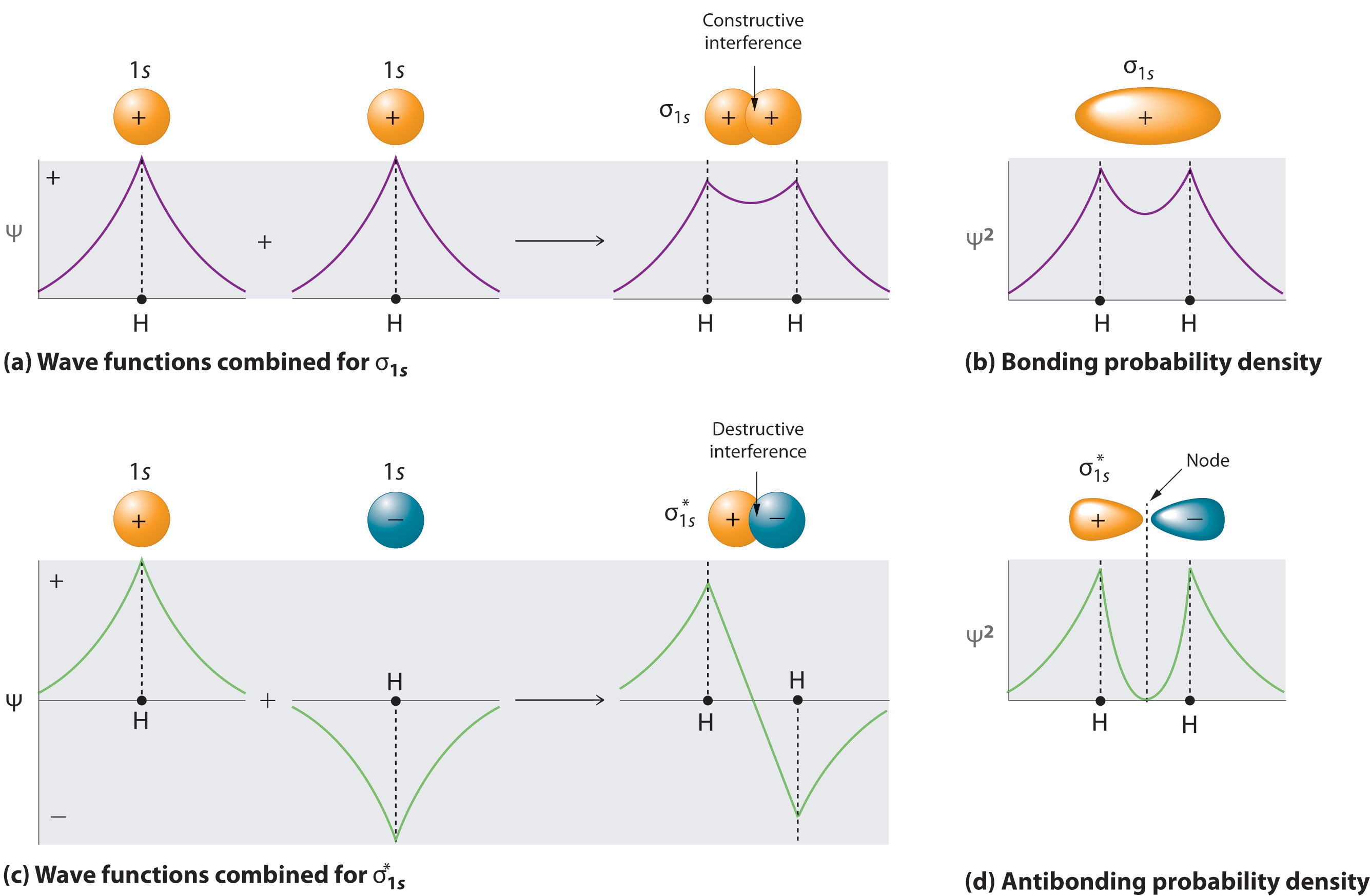
9.7: Molecular Orbitals - Chemistry LibreTexts
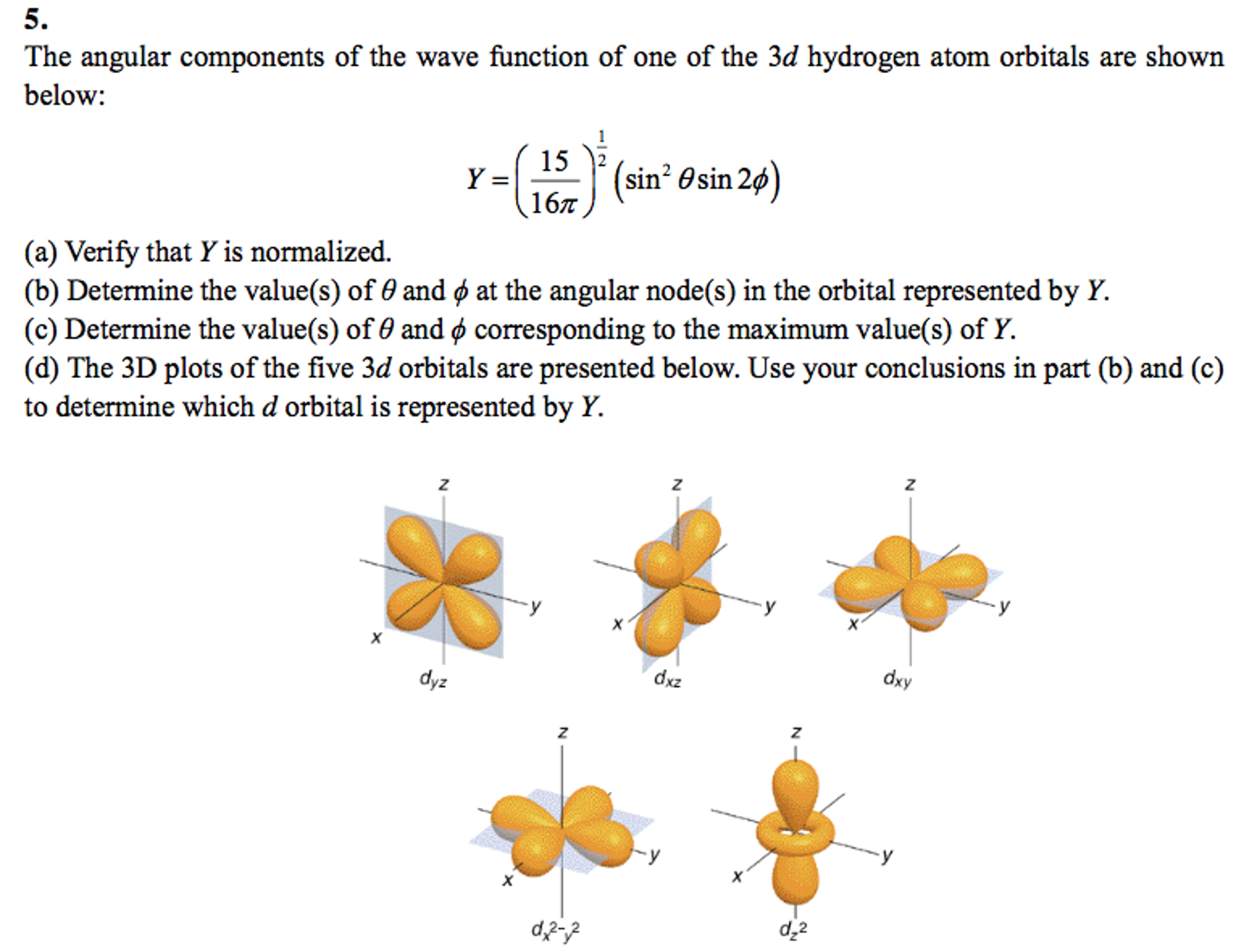
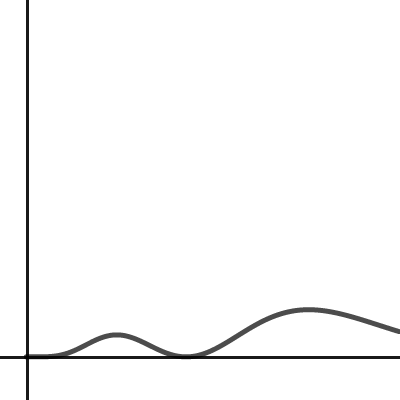
The angular components of the wave function of one of

11.10: The Schrödinger Wave Equation for the Hydrogen Atom - Chemistry LibreTexts
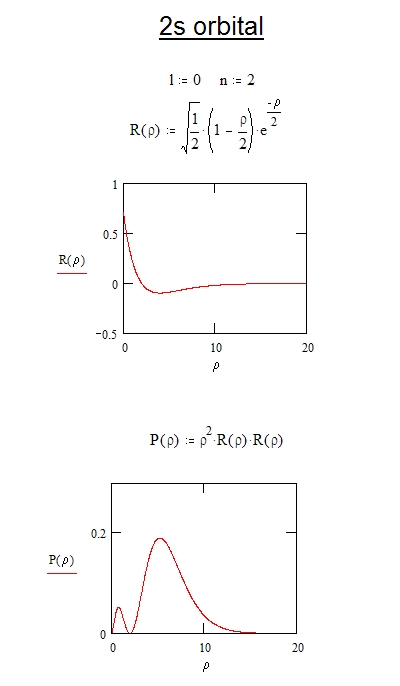
Normalization of the wave function for the electron in a hydrogen atom

The Schrodinger wave equation hydrogen atom is: Psi_{2s}= dfrac{1}{4 sqrt{2 pi}} left( dfrac{1}{a_{0}} right)^{3/2} left( 2- dfrac{r_{0}}{a_{0}} right) e^{-dfrac{r_{0}}{a_{0}}}, where a_{0} is Bohr's radius. If the radial node is 2s be r_{0}
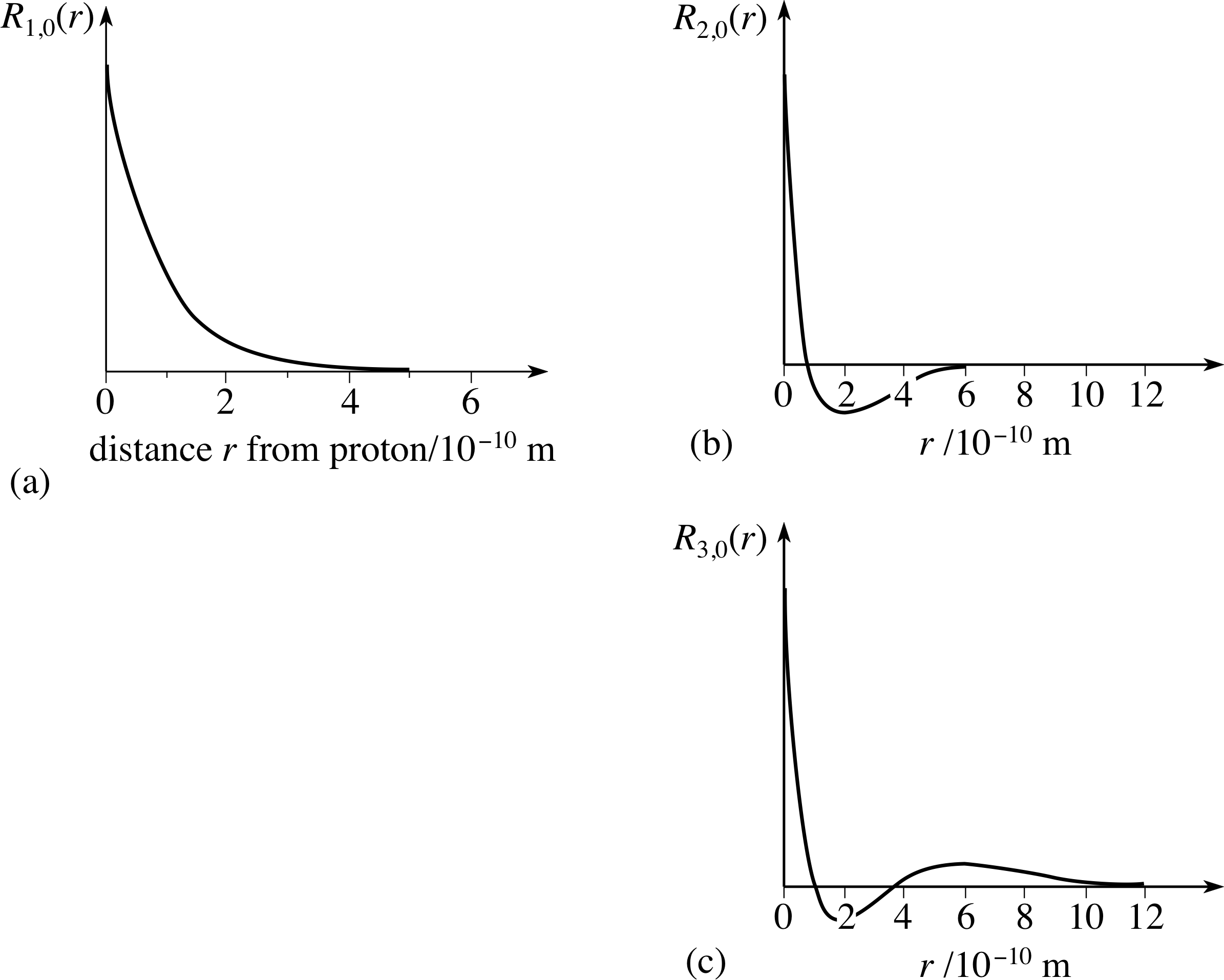
PPLATO, FLAP

Radial Wave Function and Angular Wave Functions, PDF, Atomic Orbital
Recomendado para você
-
 freetoedit#gachalife #mouth #bored #idk 😂 #remixit Ilustração de cabelo, Desenho de lábios, Cabelo de anime26 abril 2025
freetoedit#gachalife #mouth #bored #idk 😂 #remixit Ilustração de cabelo, Desenho de lábios, Cabelo de anime26 abril 2025 -
 toca life box - toca boca cute | Acrylic Block26 abril 2025
toca life box - toca boca cute | Acrylic Block26 abril 2025 -
 tela verde animação boca|Pesquisa do TikTok26 abril 2025
tela verde animação boca|Pesquisa do TikTok26 abril 2025 -
 Free Vector Hand drawn angry mouth cartoon illustration26 abril 2025
Free Vector Hand drawn angry mouth cartoon illustration26 abril 2025 -
 Not sure if this was already posted but I found this while looking for inspiration for a gacha outfit : r/WastedGachaTalent26 abril 2025
Not sure if this was already posted but I found this while looking for inspiration for a gacha outfit : r/WastedGachaTalent26 abril 2025 -
 Pin by Thinh on Lưu nhanh in 2023 Anime mouths, Cute doodle art, Anime drawing styles26 abril 2025
Pin by Thinh on Lưu nhanh in 2023 Anime mouths, Cute doodle art, Anime drawing styles26 abril 2025 -
 hecking long road by Arabella625 on DeviantArt26 abril 2025
hecking long road by Arabella625 on DeviantArt26 abril 2025 -
 CapCut_tocaboca life world26 abril 2025
CapCut_tocaboca life world26 abril 2025 -
 The Collab Together - Notability Gallery26 abril 2025
The Collab Together - Notability Gallery26 abril 2025 -
 Bocas~ Para gacha kskksks Lips drawing, Body base drawing, Eye drawing tutorials26 abril 2025
Bocas~ Para gacha kskksks Lips drawing, Body base drawing, Eye drawing tutorials26 abril 2025
você pode gostar
-
 Namorada de Cristiano Araújo pediu o cantor em casamento antes de acidente26 abril 2025
Namorada de Cristiano Araújo pediu o cantor em casamento antes de acidente26 abril 2025 -
 10 Pokémons mais fortes do Ash26 abril 2025
10 Pokémons mais fortes do Ash26 abril 2025 -
 EDUCAÇÃO FÍSICA – DIVERSÃO E CONCENTRAÇÃO COM OS JOGOS DE SALÃO26 abril 2025
EDUCAÇÃO FÍSICA – DIVERSÃO E CONCENTRAÇÃO COM OS JOGOS DE SALÃO26 abril 2025 -
 Sigma Free Fire Lite APK 1.0.0 Free Download for Android26 abril 2025
Sigma Free Fire Lite APK 1.0.0 Free Download for Android26 abril 2025 -
 Hunter X Hunter #5 Ending: Yuzu - Hyouri Ittai by Inori-saa on26 abril 2025
Hunter X Hunter #5 Ending: Yuzu - Hyouri Ittai by Inori-saa on26 abril 2025 -
Quebra Cabeça Infantil de Patrulha Canina em MDF 63 Peças 60x60cm BR_012326 abril 2025
-
 Tribe of Jones Song of Victory Lyrics26 abril 2025
Tribe of Jones Song of Victory Lyrics26 abril 2025 -
 Spider-Man developer would love to remaster the beloved26 abril 2025
Spider-Man developer would love to remaster the beloved26 abril 2025 -
 Dobrados e Canções by Banda CBMDF on Music26 abril 2025
Dobrados e Canções by Banda CBMDF on Music26 abril 2025 -
 Golden Matka play app26 abril 2025
Golden Matka play app26 abril 2025
