FDA won't comment on status of Emergency Use Authorizations for two antibody treatments
Por um escritor misterioso
Last updated 16 abril 2025

The US Food and Drug Administration told CNN Thursday morning that the agency doesn’t have any comments on the applications for Emergency Use Authorizations for Eli Lilly and Regeneron antibody treatments.
The coronavirus pandemic has brought countries to a standstill. In many places, as countries reopen, Covid-19 cases are on the rise. Follow here for the latest.
The coronavirus pandemic has brought countries to a standstill. In many places, as countries reopen, Covid-19 cases are on the rise. Follow here for the latest.

FDA won't comment on status of Emergency Use Authorizations for
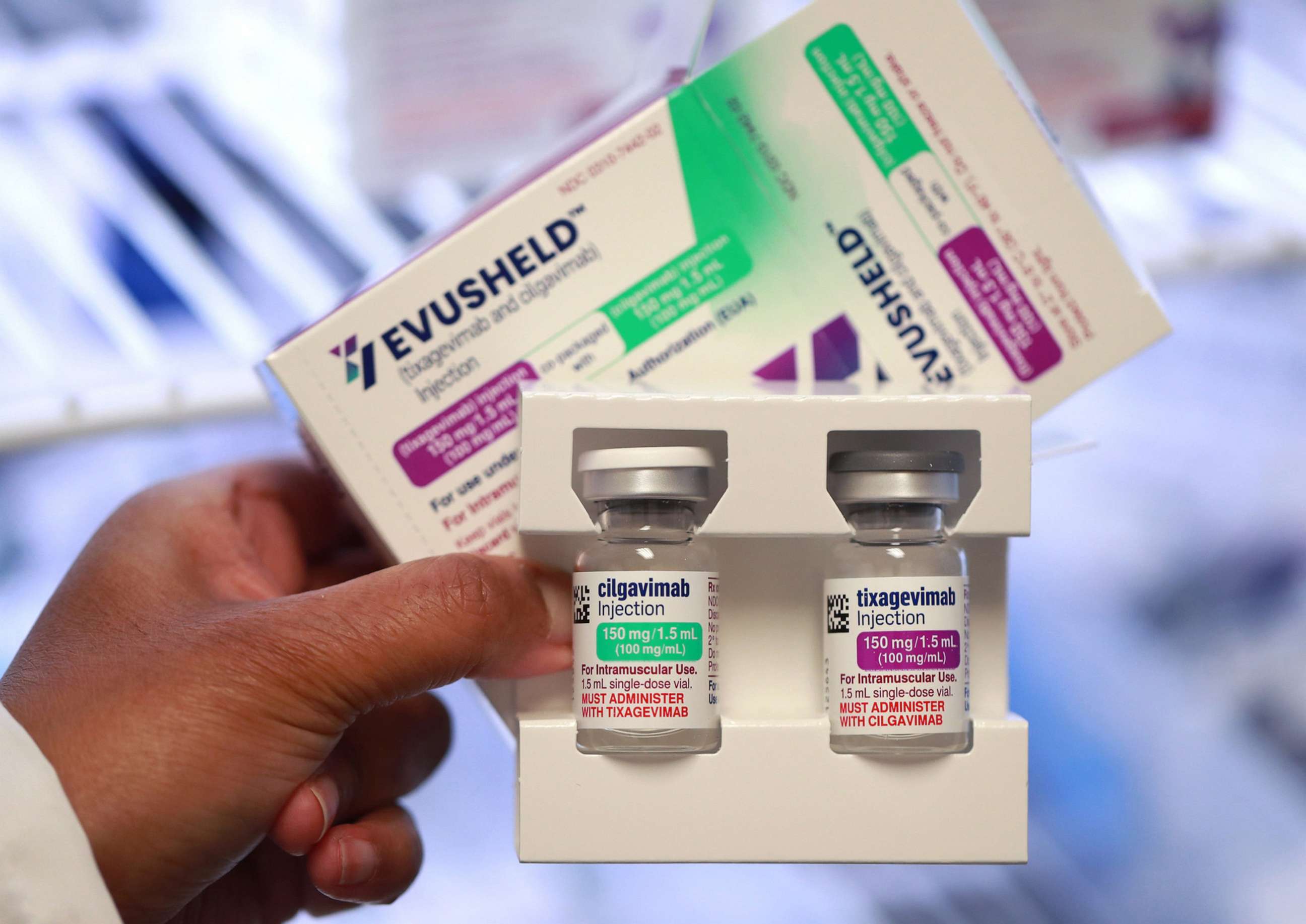
FDA withdraws emergency use authorization of COVID drug because it

FDA ends for now use of two monoclonal antibodies, spurring a halt

Emergency Use Authorization
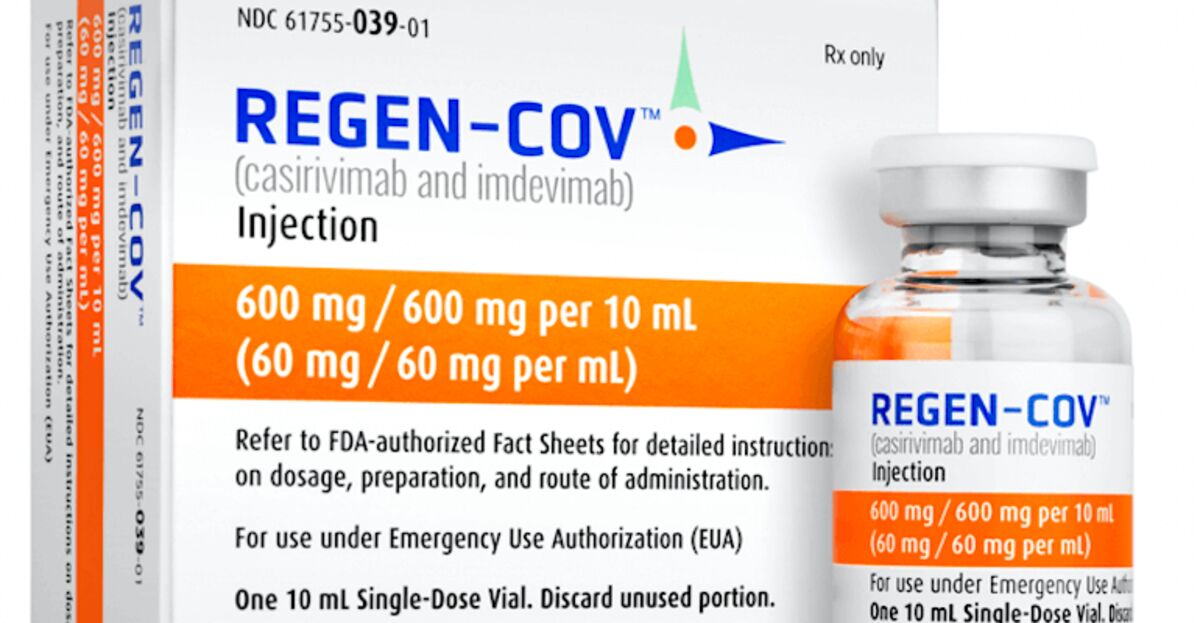
Regeneron Covid-19 Drug REGEN-COV Prevents Infections for 8 Months

Regulatory tracker: EMA backs Vertex's gene-editing therapy

US allows 1st emergency use of a COVID-19 antibody drug
FDA Meeting on COVID-19 Vaccine and Emergency Use Authorization
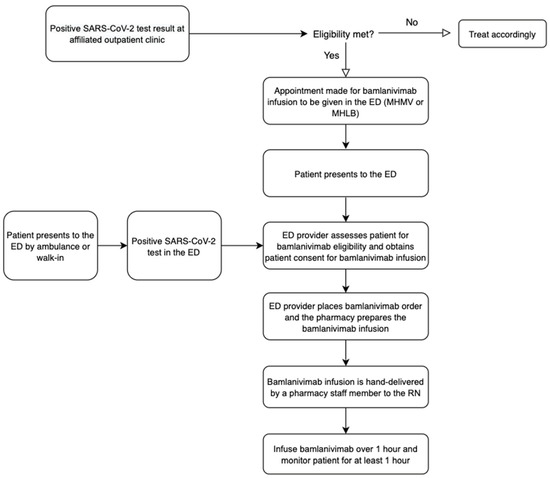
Healthcare, Free Full-Text
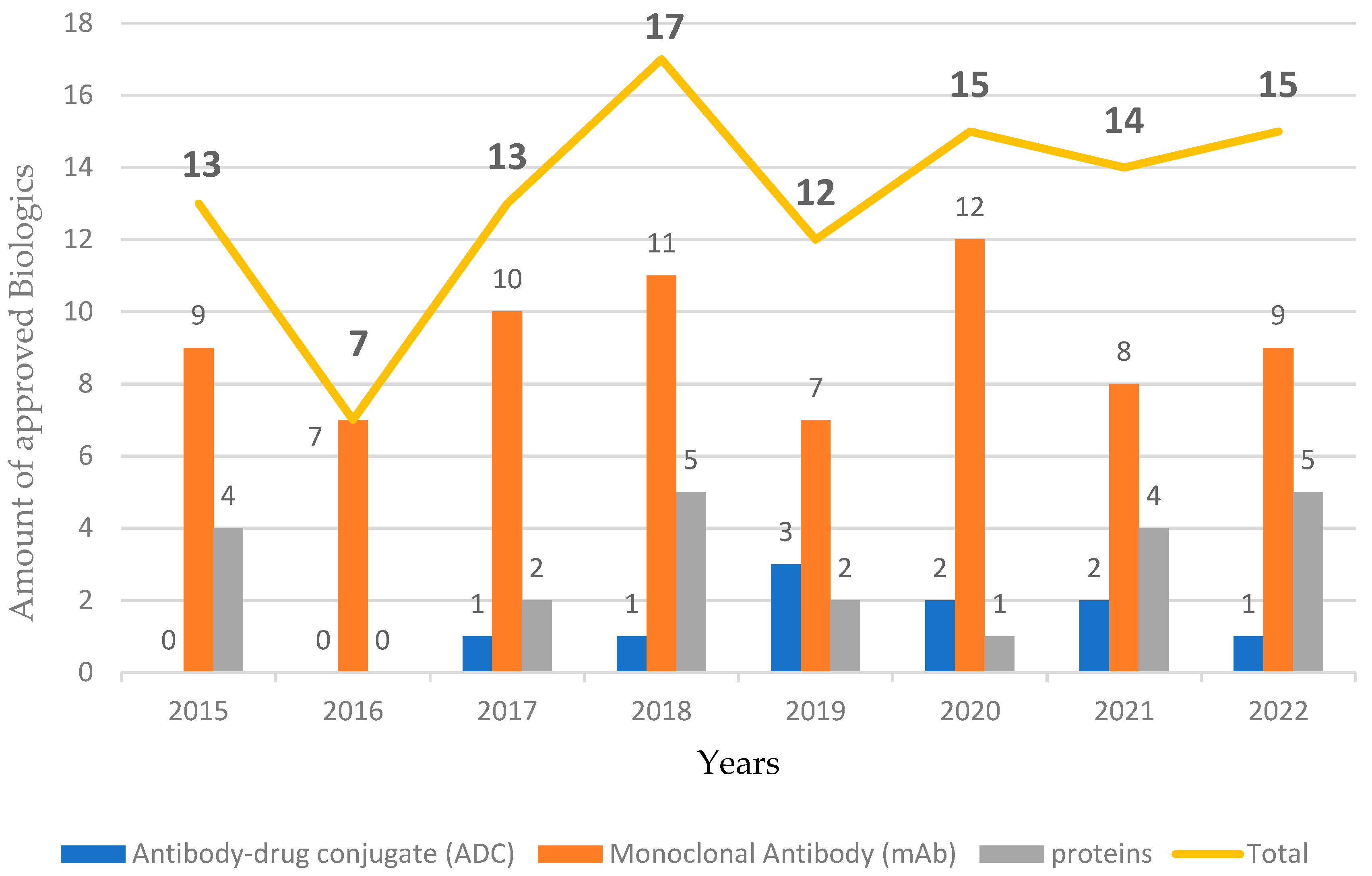
Biomedicines, Free Full-Text

Moderna asks the F.D.A. to authorize its vaccine for children
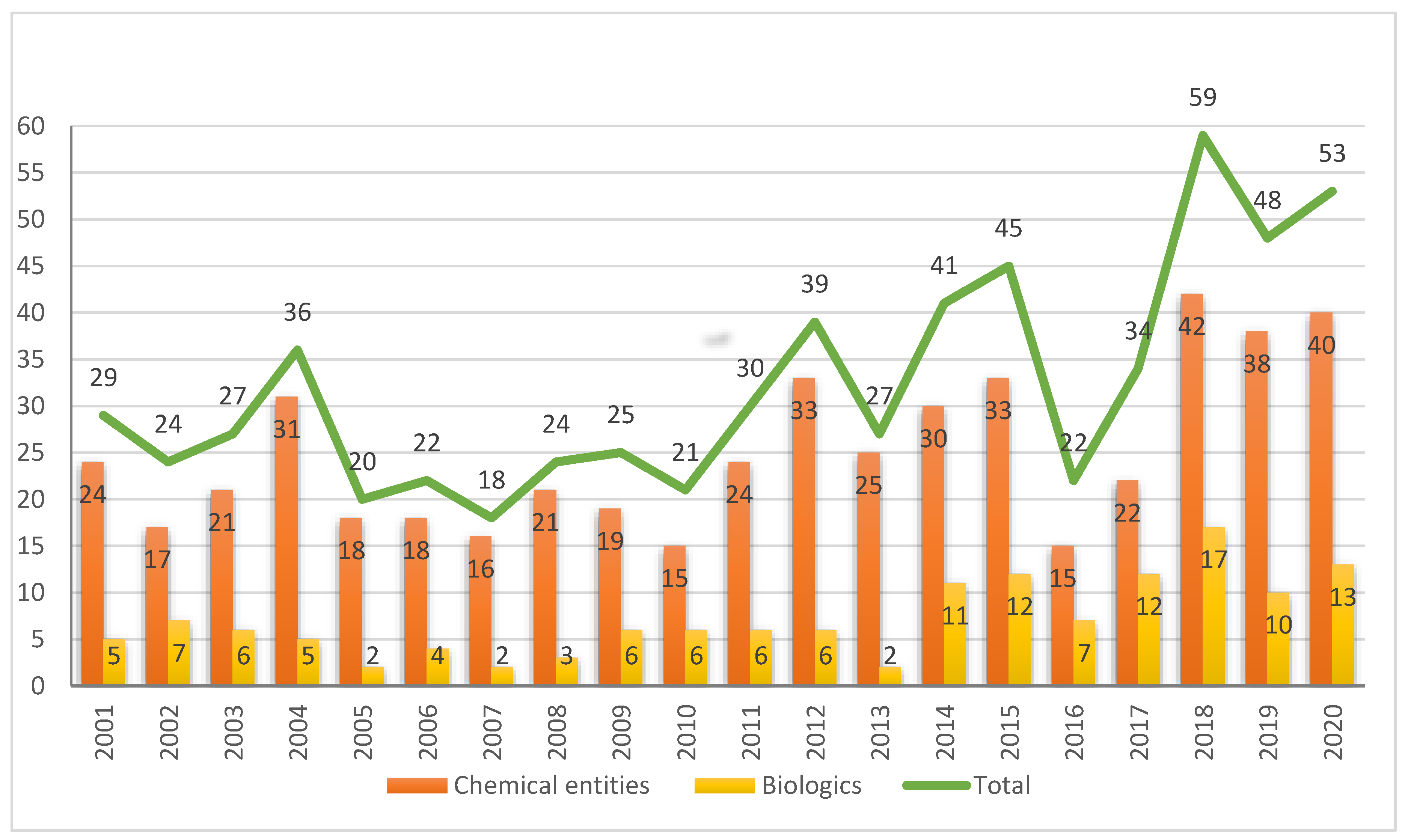
Molecules, Free Full-Text
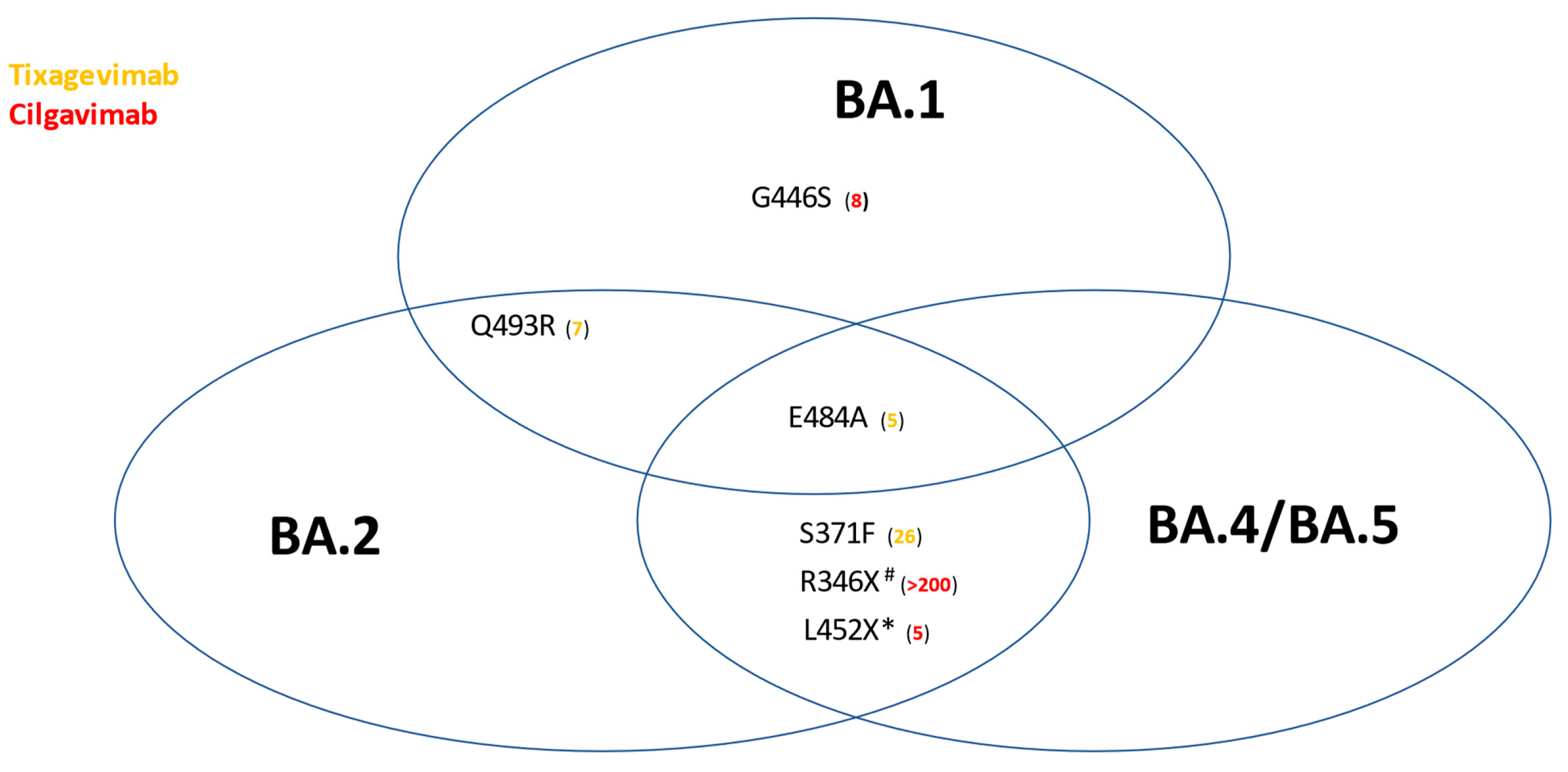
Viruses, Free Full-Text
Recomendado para você
-
 Dr. Isaac Dario Azar MD, Emergency Physician in AVENTURA, FL, 3318016 abril 2025
Dr. Isaac Dario Azar MD, Emergency Physician in AVENTURA, FL, 3318016 abril 2025 -
We continue - St. Vincent Charity Medical Center16 abril 2025
-
 Behdad David Besharatian, MD profile16 abril 2025
Behdad David Besharatian, MD profile16 abril 2025 -
 Ahmad R. Sedaghat, MD,PHD16 abril 2025
Ahmad R. Sedaghat, MD,PHD16 abril 2025 -
 Accreditation surveyors are looking at conscious sedation — Are you…16 abril 2025
Accreditation surveyors are looking at conscious sedation — Are you…16 abril 2025 -
 Johns Hopkins Defeats Franklin & Marshall and Dickinson - Johns Hopkins University Athletics16 abril 2025
Johns Hopkins Defeats Franklin & Marshall and Dickinson - Johns Hopkins University Athletics16 abril 2025 -
 Desejo de grávida” de Viih Tube vira prato em restaurante; entenda16 abril 2025
Desejo de grávida” de Viih Tube vira prato em restaurante; entenda16 abril 2025 -
 Annual Report - AugustHeart16 abril 2025
Annual Report - AugustHeart16 abril 2025 -
 Announcing Our November Book Club Selection: Home Reading Service by Fabio Morábito - Asymptote Blog16 abril 2025
Announcing Our November Book Club Selection: Home Reading Service by Fabio Morábito - Asymptote Blog16 abril 2025 -
 Safety Announcements16 abril 2025
Safety Announcements16 abril 2025
você pode gostar
-
 VOSANCO - GTA 4 - East island16 abril 2025
VOSANCO - GTA 4 - East island16 abril 2025 -
 HBO Max Greenlights Supernatural Romance 'B-Loved16 abril 2025
HBO Max Greenlights Supernatural Romance 'B-Loved16 abril 2025 -
 The Last of Us 3: Neil Druckmann CONFIRMS NEW PS5 GAME (NAUGHTY16 abril 2025
The Last of Us 3: Neil Druckmann CONFIRMS NEW PS5 GAME (NAUGHTY16 abril 2025 -
 GFL x Arma 3 : r/girlsfrontline16 abril 2025
GFL x Arma 3 : r/girlsfrontline16 abril 2025 -
 Avengers: Age of Ultron - Even Thor Can't Fight Ultron16 abril 2025
Avengers: Age of Ultron - Even Thor Can't Fight Ultron16 abril 2025 -
 Nadadora talentosa de Itupeva recebe homenagem na Câmara Municipal - Coisas de Itupeva16 abril 2025
Nadadora talentosa de Itupeva recebe homenagem na Câmara Municipal - Coisas de Itupeva16 abril 2025 -
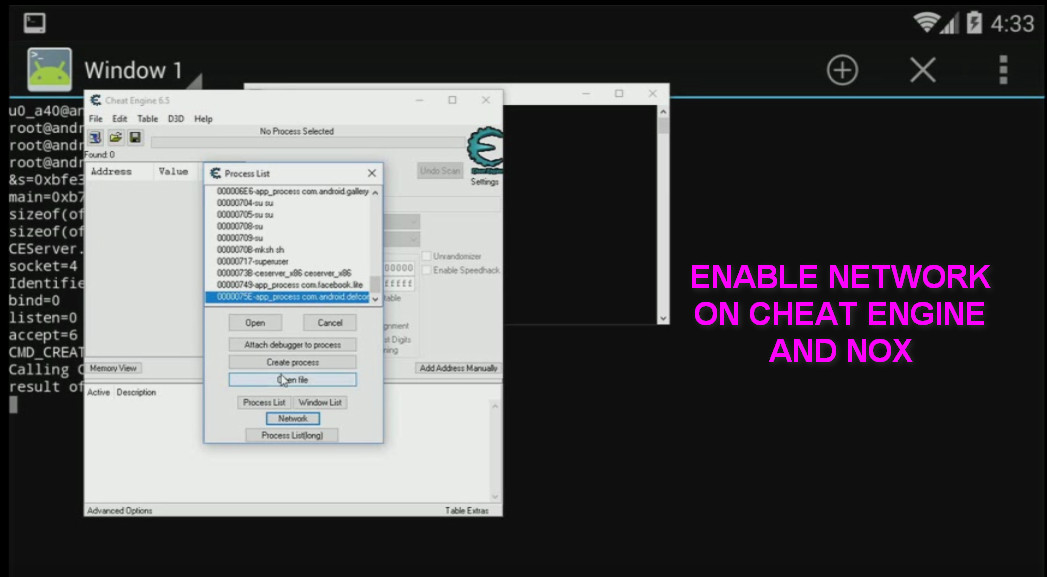 Cheat Engine Network Android PC server on NOX- Accesses and writes address16 abril 2025
Cheat Engine Network Android PC server on NOX- Accesses and writes address16 abril 2025 -
 o que significa abaixo de 2.5 na aposta esportiva,o que significa16 abril 2025
o que significa abaixo de 2.5 na aposta esportiva,o que significa16 abril 2025 -
/i.s3.glbimg.com/v1/AUTH_08fbf48bc0524877943fe86e43087e7a/internal_photos/bs/2021/5/T/03OG9MSNCqRLWpX2viyg/2016-09-08-playstation-4-pro-slim-dualshock-dual-shock-redesign.jpg) PS4 Pro e Slim terão novos controles DualShock 4 e PS Camera16 abril 2025
PS4 Pro e Slim terão novos controles DualShock 4 e PS Camera16 abril 2025 -
 Stream Naruto top ANIME music Listen to songs, albums, playlists16 abril 2025
Stream Naruto top ANIME music Listen to songs, albums, playlists16 abril 2025
