Validation of an anti-α-Gal IgE fluoroenzyme-immunoassay for the screening of patients at risk of severe anaphylaxis to cetuximab, BMC Cancer
Por um escritor misterioso
Last updated 15 abril 2025
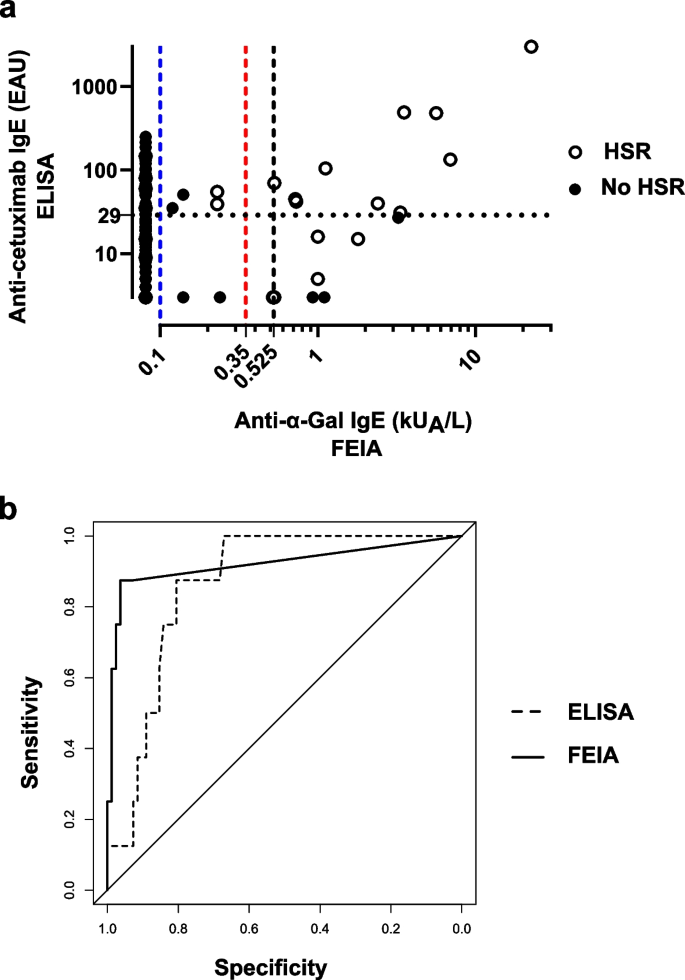
Background The link between immediate hypersensitivity reactions (HSR) following the first cetuximab infusion and the IgE sensitization against anti-galactose-α-1,3-galactose (α-Gal) is now well-established. An automated Fluoroenzyme-Immunoassay (FEIA) is available and may facilitate the screening of patients with anti-α-Gal IgE before treatment. Methods This study aimed to evaluate its performances as compared to a previously validated anti-cetuximab IgE ELISA, using 185 samples from two previously studied cohorts. Results Despite 21.1% of discrepancies between the two techniques, FEIA discriminated better positive patients and similarly negative ones with a ≥ 0.525 kUA/L threshold. Sensitivity was 87.5% for both tests, specificity was better for FEIA (96.3% vs ELISA: 82.1%). FEIA had a higher positive likelihood ratio (23.9 vs ELISA: 4.89) and a similar negative likelihood ratio (0.13 vs ELISA: 0.15). In our population, the risk of severe HSR following a positive test was higher with FEIA (56.7% vs ELISA: 19.6%) and similar following a negative test (0.7% vs ELISA: 0.8%). Conclusion Although the predictive value of the IgE screening before cetuximab infusion remains discussed, this automated commercial test can identify high-risk patients and is suitable for routine use in laboratories. It could help avoiding cetuximab-induced HSR by a systematic anti-α-Gal IgE screening before treatment.
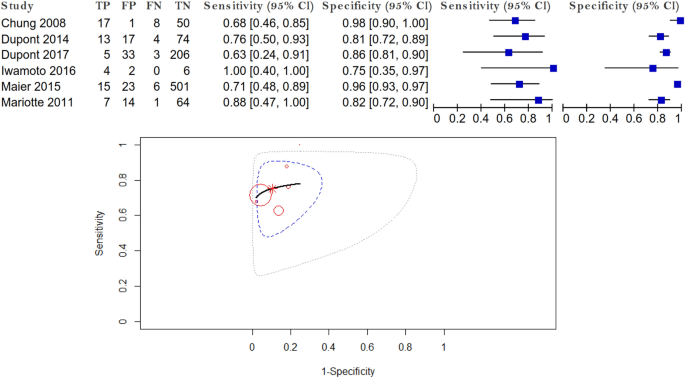
The role of IgE specific for galactose-α-1,3-galactose in predicting cetuximab induced hypersensitivity reaction: a systematic review and a diagnostic meta-analysis
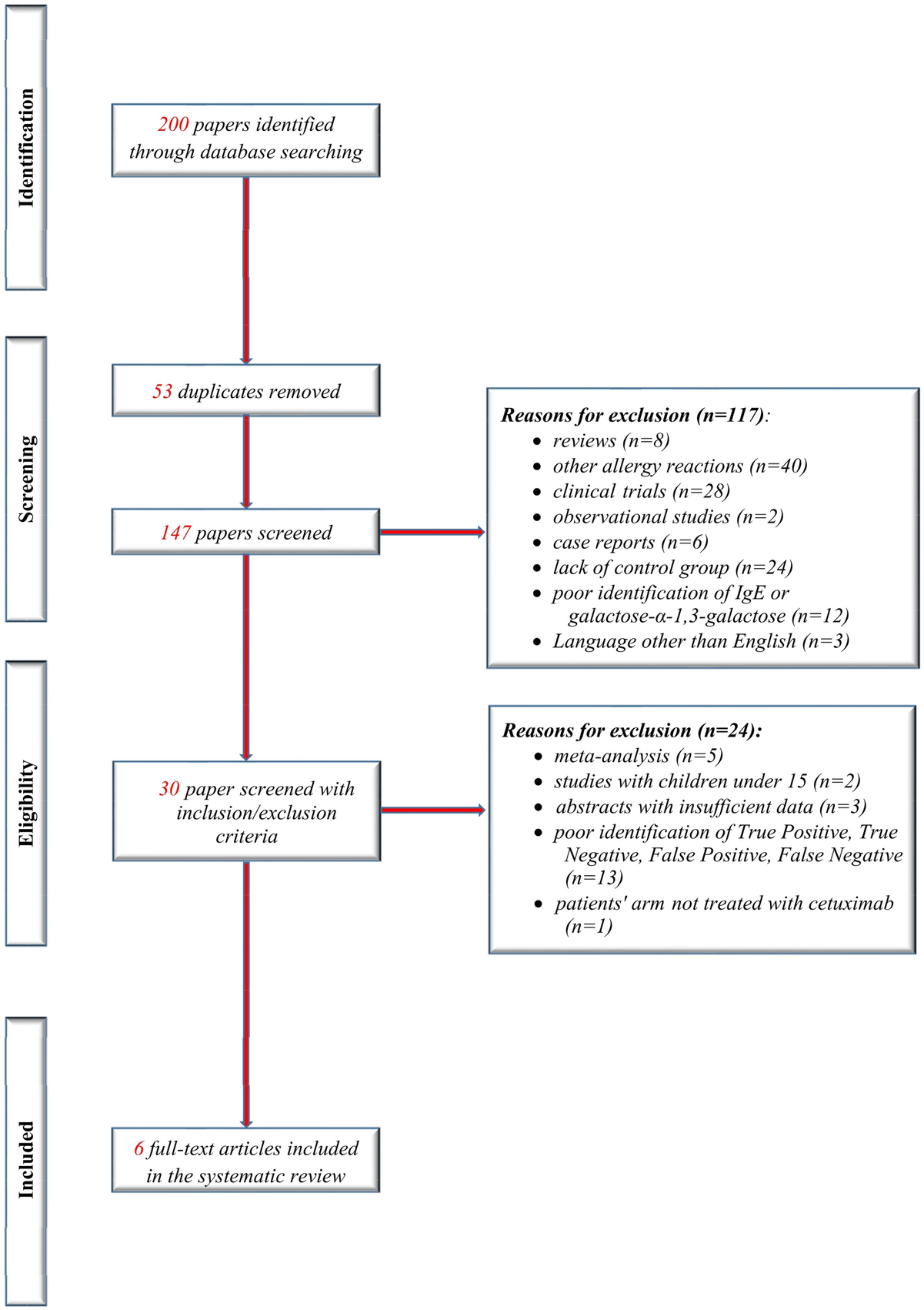
The role of IgE specific for galactose-α-1,3-galactose in predicting cetuximab induced hypersensitivity reaction: a systematic review and a diagnostic meta-analysis

Alpha-Gal-containing biologics and anaphylaxis - ScienceDirect

PDF) Alpha-Gal-containing biologics and anaphylaxis
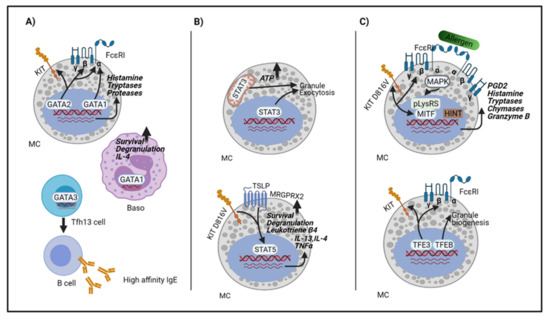
IJMS, Free Full-Text
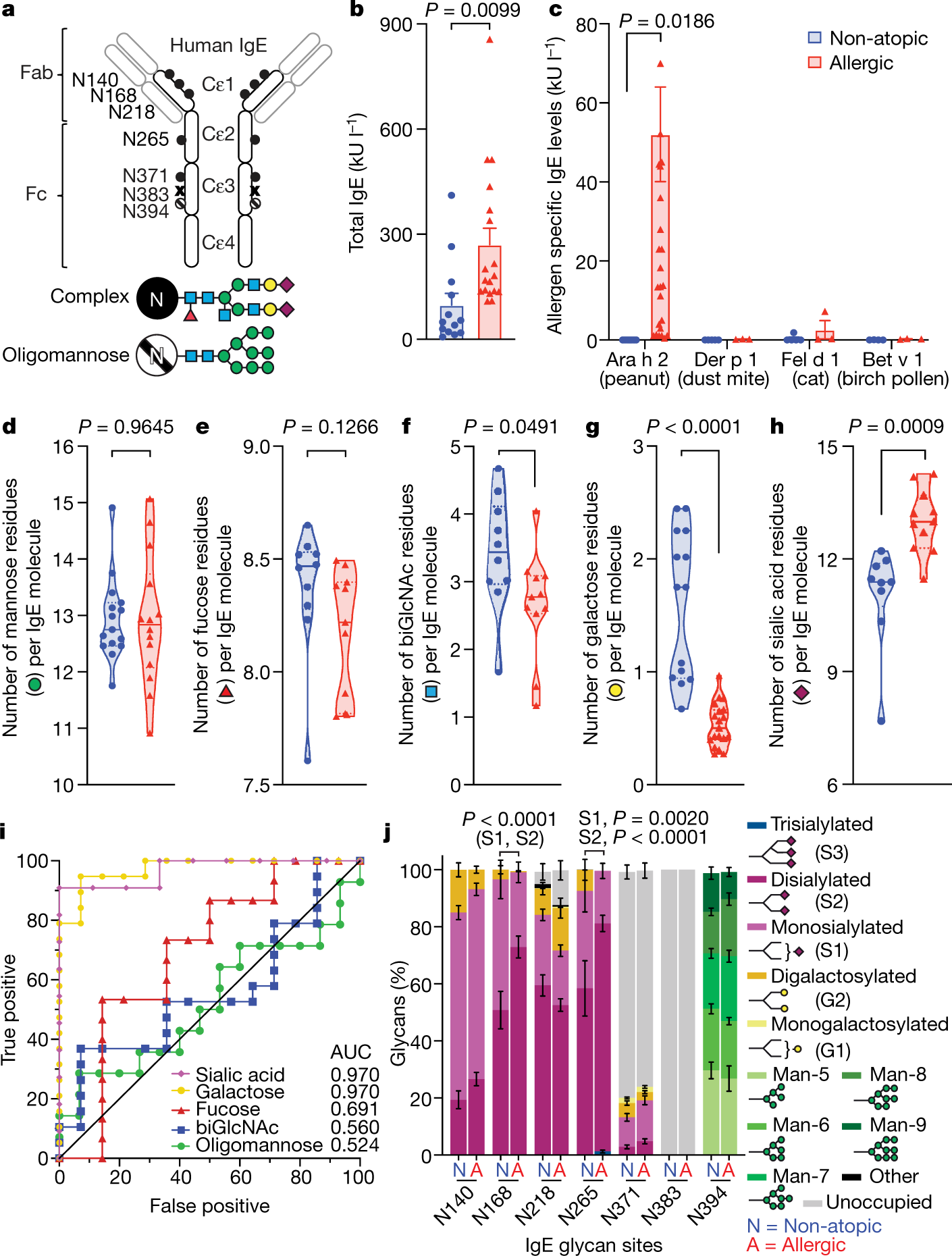
Sialylation of immunoglobulin E is a determinant of allergic pathogenicity

Delayed anaphylaxis to red meat in patients with IgE specific for galactose alpha-1,3-galactose (alpha-gal). - Abstract - Europe PMC

Study schema showing the 545 cetuximab-treated patients from whom
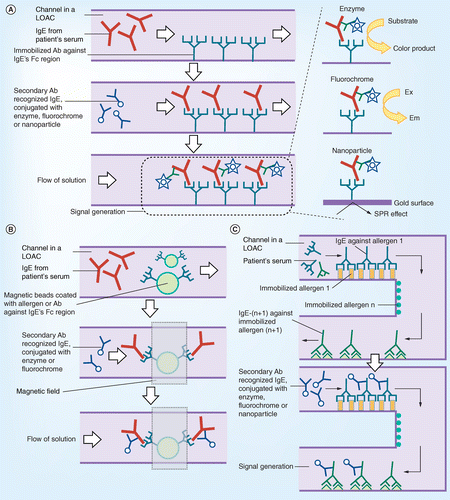
Allergen screening bioassays: recent developments in lab-on-a-chip and lab-on-a-disc systems
Recomendado para você
-
que Frozen feia eu15 abril 2025
-
 frozen feia15 abril 2025
frozen feia15 abril 2025 -
 Boneca de pano Elsa – Frozen15 abril 2025
Boneca de pano Elsa – Frozen15 abril 2025 -
 Oblee Marketplace Caixa para doces Castelo Frozen15 abril 2025
Oblee Marketplace Caixa para doces Castelo Frozen15 abril 2025 -
 Elsa, de Frozen: a rainha que resume todas as princesas Disney (e as mulheres)15 abril 2025
Elsa, de Frozen: a rainha que resume todas as princesas Disney (e as mulheres)15 abril 2025 -
 Stream tjamieres feia music Listen to songs, albums, playlists for free on SoundCloud15 abril 2025
Stream tjamieres feia music Listen to songs, albums, playlists for free on SoundCloud15 abril 2025 -
 迪士尼公主蛋糕成“车祸现场” 灰姑娘怎么斗鸡眼了?15 abril 2025
迪士尼公主蛋糕成“车祸现场” 灰姑娘怎么斗鸡眼了?15 abril 2025 -
 Receita de Bolo Confeitado, enviada por tereza cristina da silva - TudoGostoso15 abril 2025
Receita de Bolo Confeitado, enviada por tereza cristina da silva - TudoGostoso15 abril 2025 -
 Página 4 Fotos Hediondo, 300+ fotos de arquivo grátis de alta qualidade15 abril 2025
Página 4 Fotos Hediondo, 300+ fotos de arquivo grátis de alta qualidade15 abril 2025 -
 Cyntilante Produções15 abril 2025
Cyntilante Produções15 abril 2025
você pode gostar
-
 Naruto Uzumaki Fond d'ecran dessin, Naruto mignon, Coloriage manga15 abril 2025
Naruto Uzumaki Fond d'ecran dessin, Naruto mignon, Coloriage manga15 abril 2025 -
 This item is unavailable15 abril 2025
This item is unavailable15 abril 2025 -
Roblox 100 robux gift card gone from Microsoft Rewards Dashboard15 abril 2025
-
 TOP 10 - MELHORES JOGOS DE ESTRATÉGIA PARA PC15 abril 2025
TOP 10 - MELHORES JOGOS DE ESTRATÉGIA PARA PC15 abril 2025 -
 Mortal Kombat Armageddon Todos Fatalities15 abril 2025
Mortal Kombat Armageddon Todos Fatalities15 abril 2025 -
Toxic Waste Sour Candy Yellow Drum15 abril 2025
-
 Image gallery for 4 Play - FilmAffinity15 abril 2025
Image gallery for 4 Play - FilmAffinity15 abril 2025 -
 Disfarces vampiros para crianças e adultos【Entrega em 24h】15 abril 2025
Disfarces vampiros para crianças e adultos【Entrega em 24h】15 abril 2025 -
 Naruto shippuden blu-ray releases question : r/Naruto15 abril 2025
Naruto shippuden blu-ray releases question : r/Naruto15 abril 2025 -
 Jogo de estratégia em tempo real Ancestors Legacy será lançado no15 abril 2025
Jogo de estratégia em tempo real Ancestors Legacy será lançado no15 abril 2025